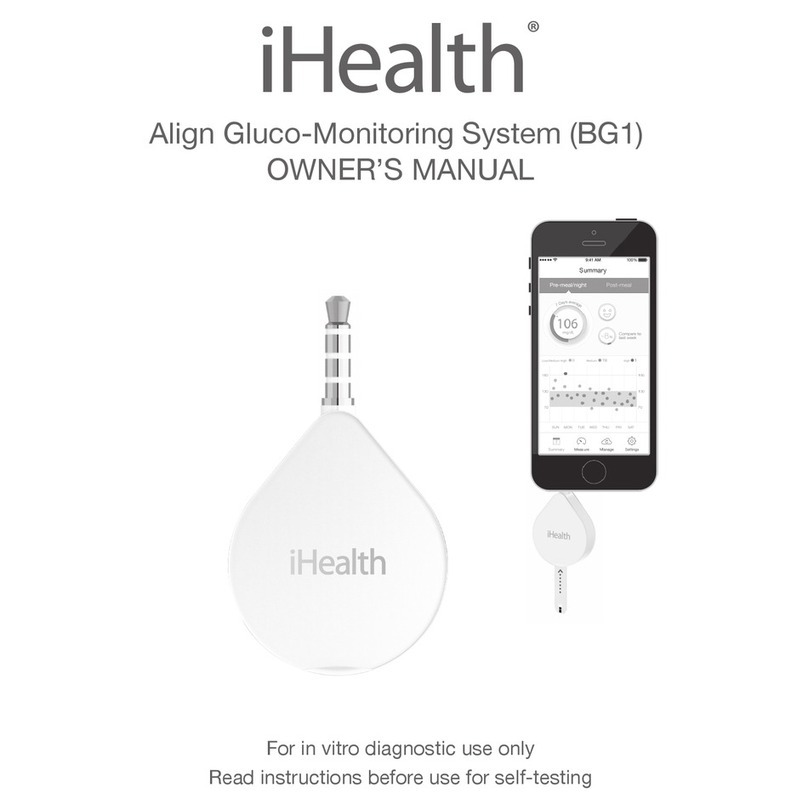
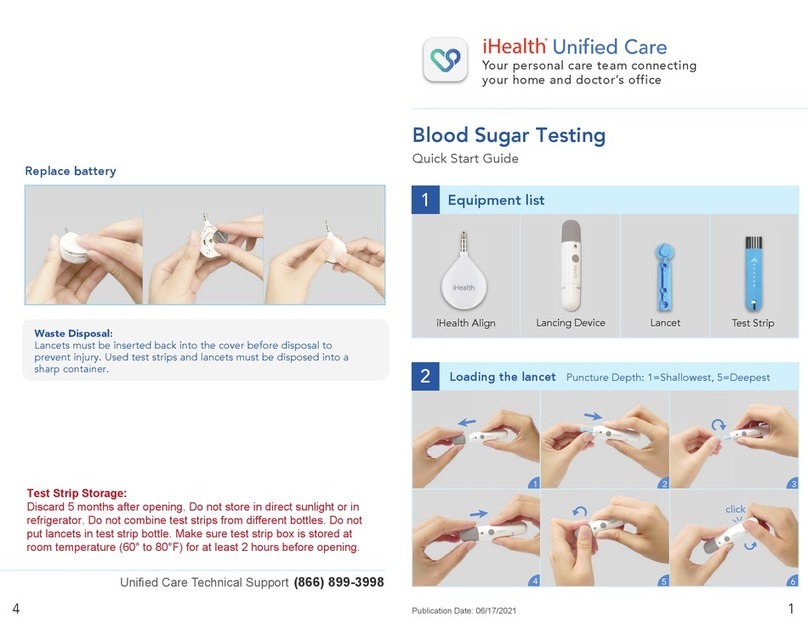
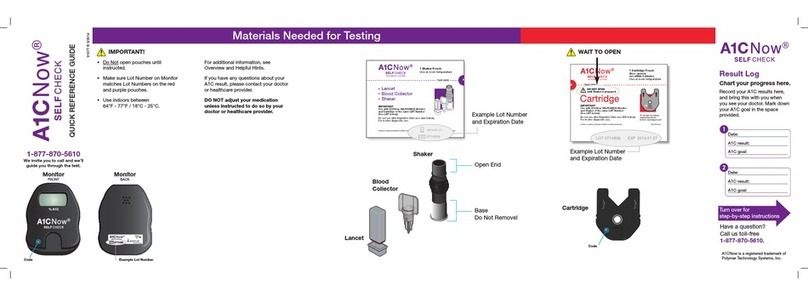
Table of Contents
INTRODUCTION............................................................................................................................1
INTENDED USE.............................................................................................................................1
IMPORTANT SAFETY INSTRUCTIONS....................................................................................1
LIMITATIONS OF USE ..................................................................................................................2
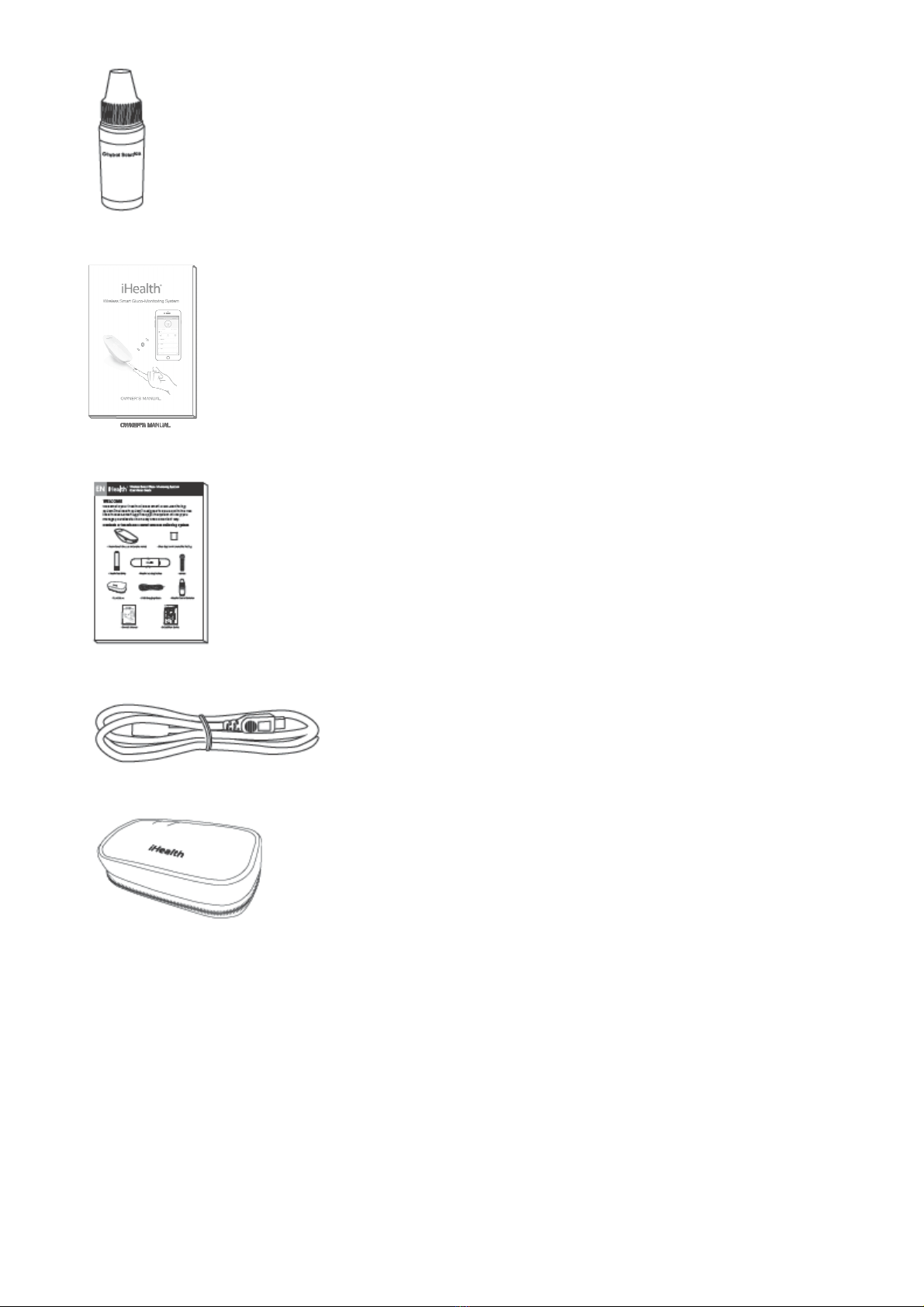
CONTENTS OF THE WIRELESS SMART GLUCO-MONITORING SYSTEM.....................4
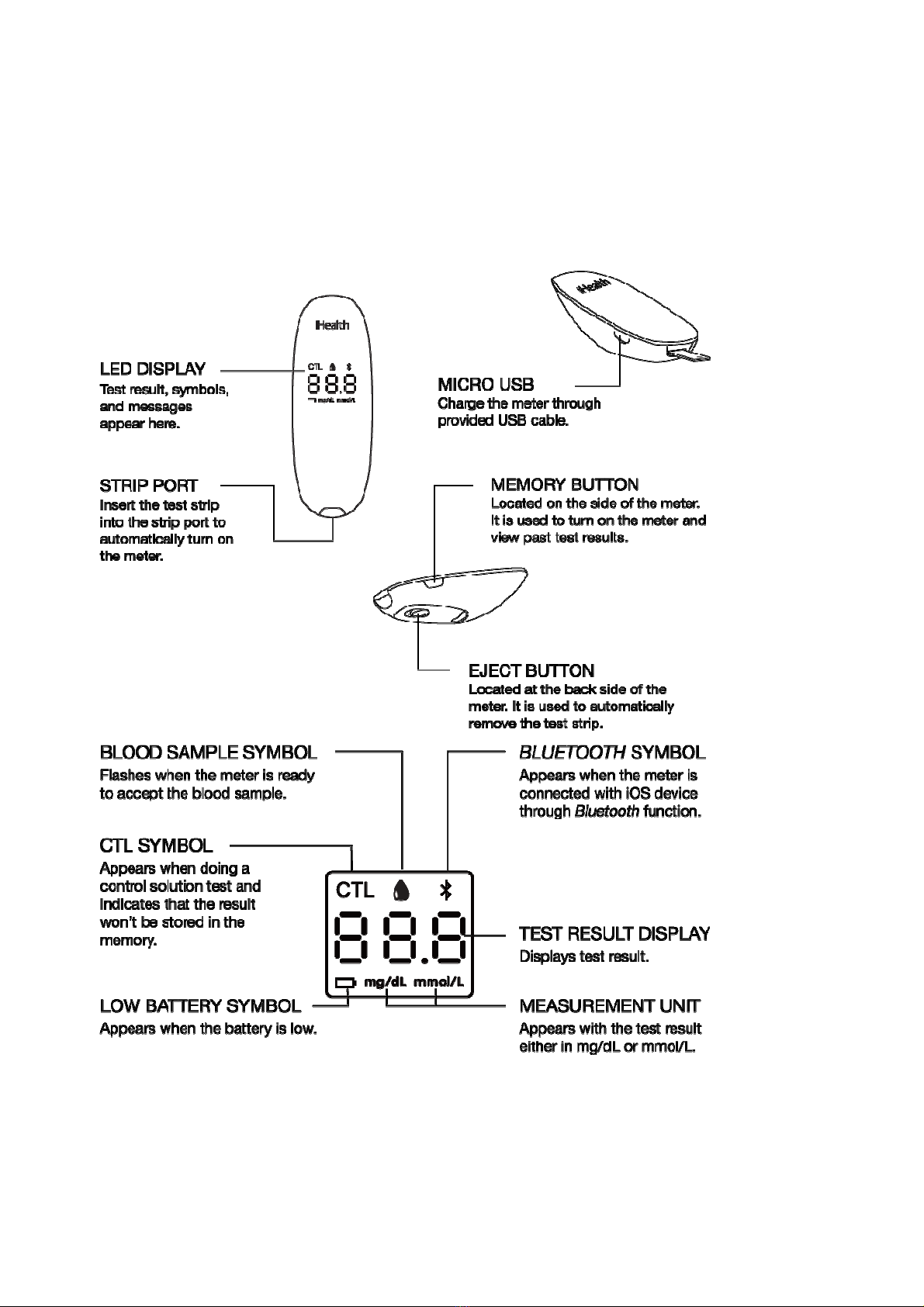
Parts and Displays .....................................................................................................................6
Mobile Device Compatibility....................................................................................................7
TEST PRINCIPLE ..........................................................................................................................8
IMPORTANT TEST INFORMATION............................................................................................8
TEST BLOOD GLUCOSE LEVEL ...............................................................................................8
DATA SYNCING ...........................................................................................................................13
REVIEWING SAVED TEST RESULTS ON THE METER......................................................13
CLEANING AND DISINFECTION..............................................................................................13
SIGNS OF POTENTIAL PHYSICAL AND PERFORMANCE DETERIORATION...............14
INFORMATION ABOUT ALTERNATIVE SITE TESTING (AST) ...........................................14
What Is Alternative Site Testing?............................................................................................14
What Is the Advantage of Alternative Site Testing?................................................................15
When Should You Use Alternative Site Testing? ....................................................................15
IMPORTANT INFORMATION ABOUT CONTROL SOLUTION TESTS...............................16
PERFORMING A CONTROL SOLUTION TEST .....................................................................16
Out-of-Range Results..............................................................................................................17
COMPARING GLUCOSE METER TEST RESULTS WITH LABORATORY RESULTS ....18
Before the Lab Test .................................................................................................................18
While at the Lab......................................................................................................................18
THE iHealth Wireless Smart Gluco-Monitoring System SPECIFICATIONS .......................18
MAINTENANCE AND STORAGE OF YOUR METER............................................................19
SYSTEM TROUBLESHOOTING...............................................................................................19
Display Messages....................................................................................................................19
Troubleshooting ......................................................................................................................21
WARRANTY INFORMATION .....................................................................................................22
EXPLANATION OF SYMBOLS..................................................................................................23
IMPORTANT INFORMATION REQUIRED BY THE FCC......................................................24
ELECTROMAGNETIC COMPATIBILITY INFORMATION .....................................................26