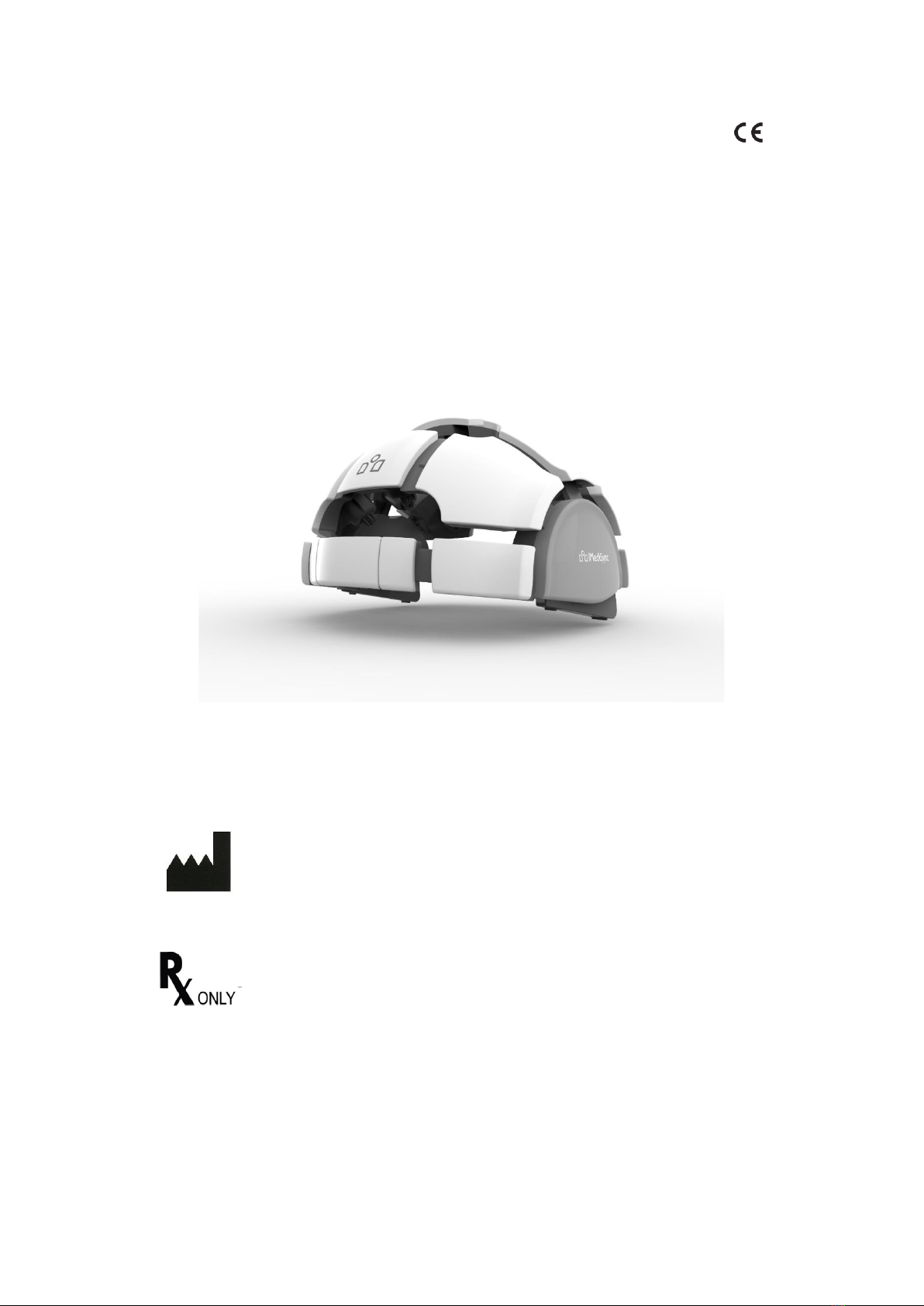
Page 8 of 46
device and/or components shall not be used and be requested for repair service. If the
amplifier is damaged, please contact the manufacturer or the service center.
7. Safety precautions to avoid hazards in the event of inadequate hygiene or if the subject
contacts the device contaminated with toxic substance:
If the device’s hygiene is inadequate, clean the device with alcohol. If the device is
contaminated by toxic substance, stop using the device and contact the manufacturer or the
service center.
8. Inadequate EEG recordings or inaccurate interpretation of EEG can be caused by the
following: user’s inadequate ability to operate the device , inaccurate placement of electrodes,
electromagnetic interference, problem with computer device, use of alternative software
and/or operating system not provided by the manufacturer. The User operating the
iSyncWave device shall have adequate knowledge and experience in EEG measurement and
the device.
9. Before wearing the device, please turn on the device for the safety of the patient/subject.
10. Before taking off the device, please turn off the device for the safety of the patient/subject.
11. Do not modify this equipment without authorization of the manufacturer.
12. Do not use the device with HF surgical equipment to protect the patient against burns.
13. Do not use the device with defibrillator.
14. The conductive parts of electrode should not contact other conductive parts including earth.
3.2 Precautions
1. Read all instructions and labels including this manual before starting to use the device system.
2. Do not attach or detach any device components while the device is on to prevent any
damage to the system or components. Turn off the device when attaching or detaching any
components to the device.
3. Do not use acetone or any other cleaning solvents to clean the device.
4. The battery life of the device may be shortened if the device is used frequently and/or for a
prolonged period of time.
5. The battery life of the device is affected by how often you charge and discharge the battery.
6. The battery life and capacity may decrease when the device is stored in a high-temperature
environment.
7. The battery may self-discharge when the device is in storage.
8. Keep the battery charged. If the battery is discharged, it may take a longer time to fully charge.
9. Do not immerse the device into any liquid.
10. Do not expose the device to direct sunlight, heat source of thermal radiation, moisture, vibration,
mechanical shock, excessive dust, or humidity.
11. The warranty will be void if the device is opened, disassembled, or altered by any unauthorized
personnel.
12. Do not use when the device is damaged.
13. Do not use when the device is wet. If any moisture penetrates the device, have the device