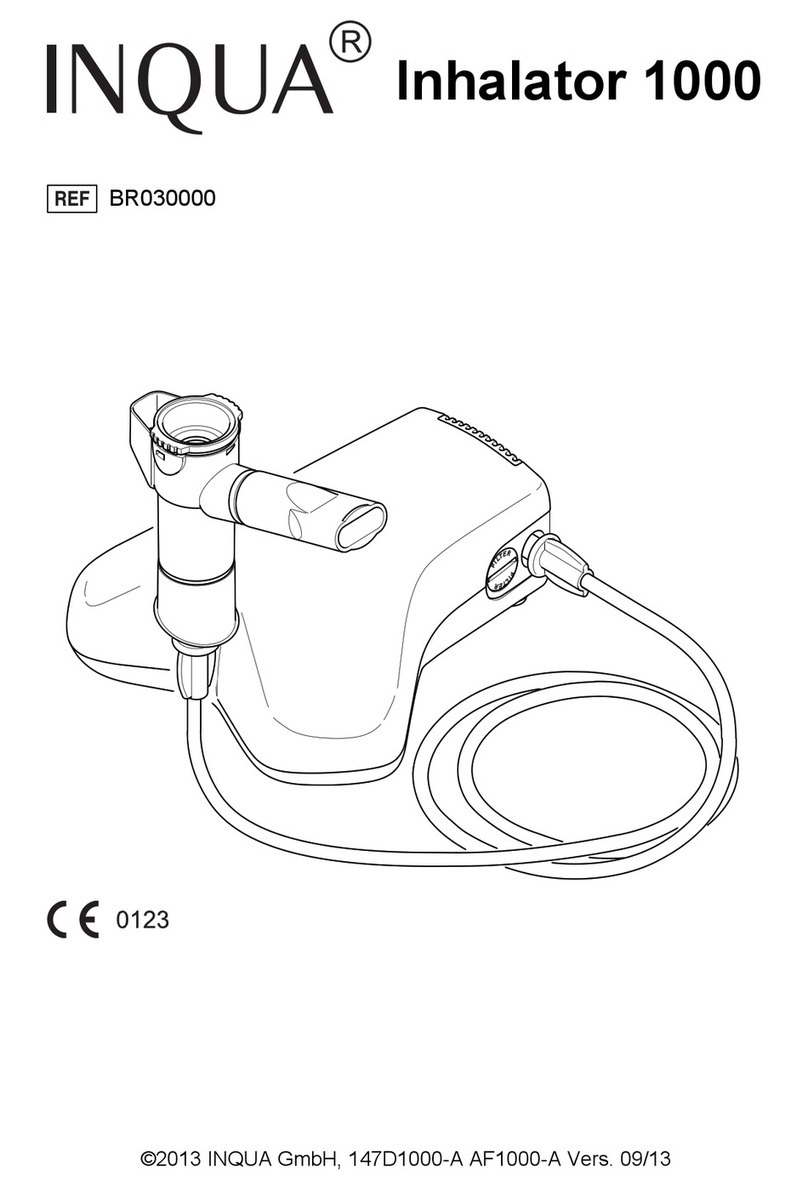
InfoBionic MoMe 01854 User manual

MoMe® Kardia System Patient Guide

page 2
10135 revI
InfoBionic’s MoMe® Kardia System may be covered under one
or more United States and International Patents. Please see the
website: www.infobionic.com to satisfy the virtual patent
marketing provisions including the America Invents Act and 35
U.S.C. § 287(a). Additional patents may be issued or pending in
the US and elsewhere.

page 3
Table of Contents
MoMe® Kardia System Components ................................................. 4
Unpacking MoMe® Kardia Device ..................................................... 5
Monitoring ......................................................................................... 6
Device Controls ................................................................................ 7
Notifications ...................................................................................... 8
Belt Clip ............................................................................................ 9
Charging Battery Pack ......................................................................... 9
Installing Battery Pack ....................................................................... 10
Removing Battery Pack ...................................................................... 11
Warnings ........................................................................................ 12
Cautions ......................................................................................... 13
Battery Information ......................................................................... 14
System Specifications ..................................................................... 15
Electromagnetic Emissions Compliance ......................................... 16
SAR Exposure Information ............................................................. 19

page 4
MoMe® Kardia System Components
MoMe® Kardia Kit Components
No.
Component Description
1
Monitor Small, lightweight battery operated device that
collects, stores, and transmits physiological data
to the remote server via built in cellular module
1
Lead Set Attaches to the MoMe® Kardia monitor and
to each electrode
1
Belt Clip Used to carry the MoMe® Kardia monitor on
your belt or waistband during the day
2
Rechargeable
Battery Pack
Battery packs to power the MoMe® Kardia monitor.
A fully charged battery will last for 24 hours and
must be replaced with a charged battery each day
to ensure uninterrupted monitoring
1
Charger Dock Used to charge the battery packs
1
Charger Dock
Power Cord Used to connect the charger dock to AC wall socket
-
Electrodes The lead set snaps to the electrodes.
Electrodes will be provided by your physician
Inspect all parts before use to ensure nothing is damaged and/or missing.

page 5
Unpacking MoMe® Kardia Device
Unpack the MoMe® Kardia Kit and locate components
listed in the Kit Components chart above.
Plug in the charger dock and place one of the
two included battery packs in the charger.
Insert the second battery pack into the back of
the Monitor (for more detailed instructions, see
diagram on page 10). The device should turn on.
Attach the lead set to the monitor.
Place electrodes as indicated on page 6.
You are now monitoring.
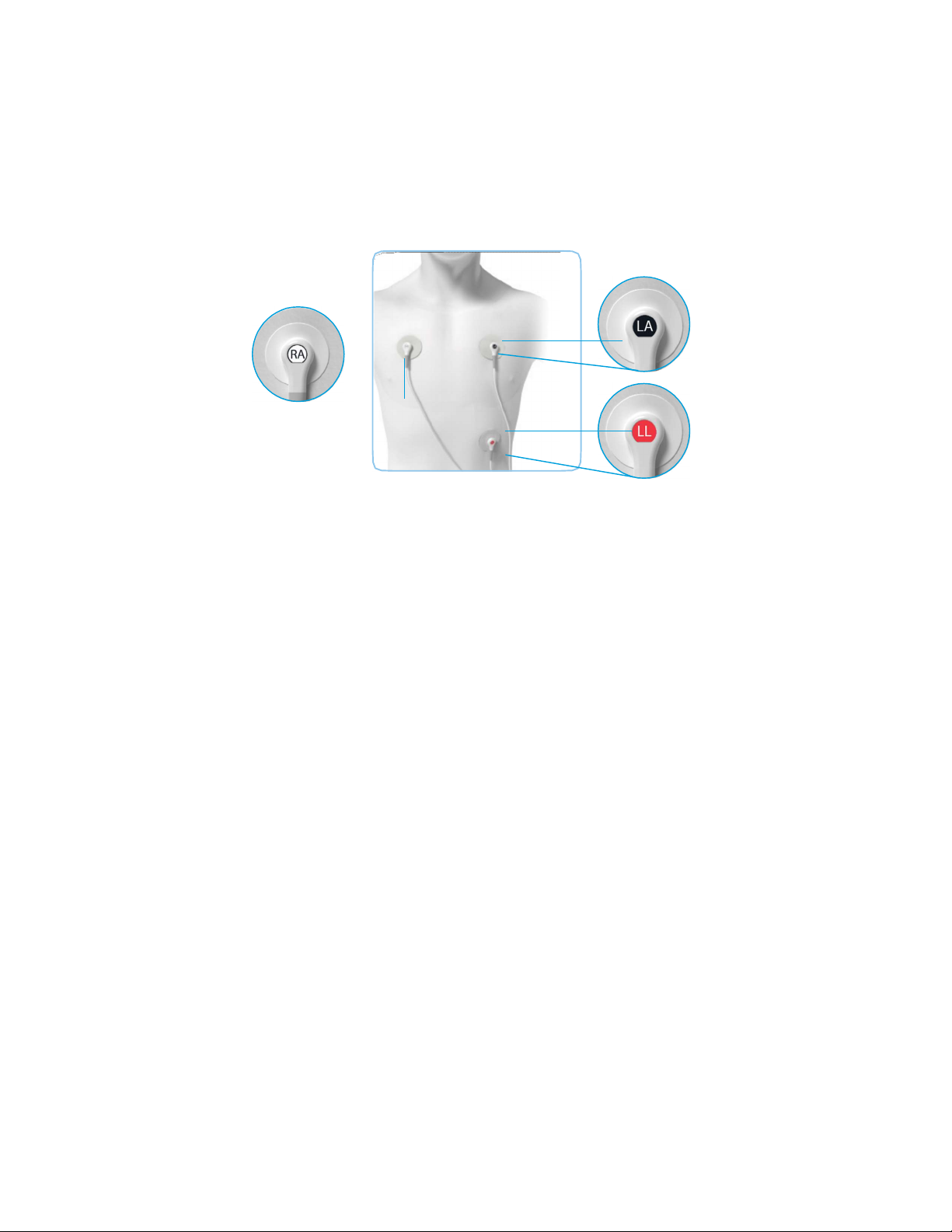
page 6
Monitoring
Once the monitor is activated and operating, the system requires
no intervention to capture or analyze data. However, your physician
should instruct you about the following responsibilities:
•
Charge the battery pack every day
•
Report any symptomatic events by pressing the event
record button as instructed by your physician
•
Return the system to the practice at the end of monitoring
Front of body

page 7
Device Controls
The MoMe® Kardia monitor has three buttons:
Event Record, Wake, and Volume.
Wake Button
Volume
Button
Event Record
Button
Wake: Press the wake button at any time to display the current battery
level status.
Volume: Press the volume button to adjust the volume or silence alerts
on the monitor. To adjust the volume, press up or down on the volume
button. The display will be updated with the current level and a tone
will be played at the current volume for audible feedback of the volume
level. The lowest volume setting mutes the speaker and can be used
when the patients sleep should not be disturbed. Pressing the volume
button one more time after the lowest volume setting sets the monitor to
vibrate mode.
Volume Up
Volume Down
Vibrate

page 8
Device Controls continued
Event Record: Press the event record
button located on the face of the
device to report a symptomatic event.
The screen will display a solid heart
when the event has been recorded.
Press and hold the Event Record
Button for 3 seconds to record
Notifications
Low Battery: When the battery level
is low, the device will play an audio
alert and will display the BATT LOW
notification shown below.
When the battery level is low the screen
will display BATT LOW

page 9
Belt Clip
The MoMe
®
Kardia Monitor must
be used with the included belt clip.
Slide the monitor up and into the belt
clip holster to secure the monitor
in place then slide the clip over
your belt or waistband with the lead
set attachment facing upwards.
Charging Battery Pack
To charge the Battery Pack:
•
Slide the battery pack into the charger dock until the
charging indicator turns red and the battery pack stops.
•
The battery pack is fully charged – when the indicator light
turns green.
Note: Each battery pack takes approximately 3 hours to fully charge.
Insert battery Charge in progress Charge complete
Red
Green
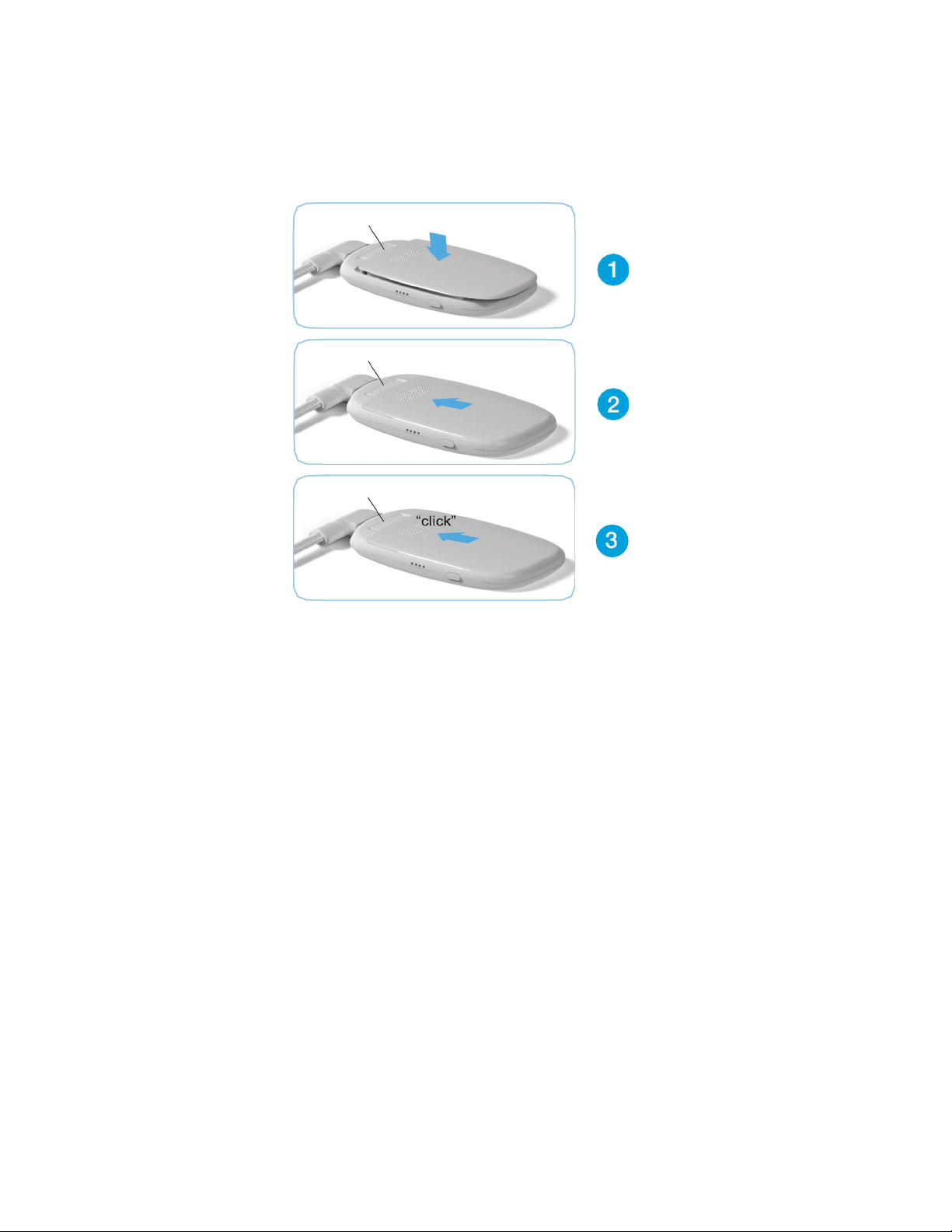
page 10
Installing Battery Pack
To install the Battery Pack, follow these steps:
1. Place the battery pack into the monitor, pushing down
until it is fully seated.
2. Slide the battery pack towards the slide lever lock.
3. Battery is installed when you hear the slide lever lock “click”.
Slide Lever Lock
Slide Lever Lock
Slide Lever Lock

page 11
Removing Battery Pack
To remove the Battery Pack, follow these steps:
1. Push the slide lever lock to the right and hold.
2. Push the battery pack away from the slide lever lock
and release the slide lever lock.
3. Lift the battery pack up and remove from monitor.
Slide Lever Lock
Slide Lever Lock
Slide Lever Lock

page 12
Warnings
1. Warning: MoMe® Kardia is not intended for use on infants weighing less
than 10kg (22 lb).
2. Warning: Use only specified MoMe® Kardia cables and accessories. Use of
any other cables and accessories may negatively affect EMC performance
resulting in increased emissions and decreased immunity.
3. Warning: Use only specified MoMe® Kardia accessories. Use of any other
accessories may result in non-compliance.
4.
Warning:
To avoid possible strangulation, route all cables away from the
patient's throat.
5. Warning: The MoMe® Kardia Device is not defibrillation-proof. Remove MoMe®
Kardia Device and disconnect patient leads before external defibrillation.
6. Warning: Do not service or repair any components of the MoMe® Kardia
system. Removal or tampering of the lead wires or any other component
may alter device performance and cause device malfunction or failure.
Contact your physician’s office for product repair or replacement.
7. Warning: The MoMe® Kardia system may be affected by equipment even
if the equipment is CISPR compliant.
8. Warning: The MoMe® Kardia system should not be used in the presence
of flammable anesthetics.
9. Warning: The MoMe® Kardia contains a cellphone. If the patient has an
implantable device, they should be instructed to follow their implantable
device manufacturer’s recommendations for use with a cellphone.
10. Warning: Never attempt to repair or service any MoMe® Kardia equipment.
Repairs by untrained, unauthorized individuals may damage the equipment
or cause system malfunction.
11. Warning: The MoMe® Kardia is not intended to be used in an Oxygen rich
environment.
12. Warning: Electrodes may cause skin irritation or breakdown. We recommend
that standard FDA approved Ag/AgCL ambulatory monitoring electrode
patches be used and that the patient be instructed on what to do if skin
irritation occurs.
13. Keep the system out of reach of children and pets

page 13
Cautions
1.
MoMe® Kardia uses cellular phone technology, so the system operation and
data transmission may be affected or interrupted by poor cellular coverage or
electromagnetic interference. If data transmission is interrupted, MoMe® Kardia
will automatically cache the data until cellular coverage or communication
between the two devices is restored and then send the stored data.
2.
Use only MoMe® Kardia parts and accessories with the MoMe® Kardia system.
Using non-MoMe® Kardia equipment may result in system malfunction or failure.
3.
Use only with the supplied battery packs, charger dock, and power cord.
4.
Prior to setting up a new patient with MoMe® Kardia, carefully inspect all
system components for defects or damage. Check lead wires for cracks
or fraying in the wiring, and cracks around the connector and snap leads.
Do not use the MoMe® Kardia system if any component appears defective,
damaged, or worn (e.g. cracks, dents, chips, cuts, kinks, or crushed or
elongated sections), as this may result in system malfunction or failure.
Contact your physician’s office for a replacement, if needed.
5.
MoMe® Kardia is not waterproof:
— Protect all MoMe® Kardia parts from water, liquids or moisture which will
damage equipment and affect system operation;
— Do not immerse any part of the MoMe® Kardia system in water or fluids.
Do not spray device with cleaners or other liquids;
— Never bathe, shower, or swim while wearing the MoMe® Kardia Device
(while bathing or swimming, store MoMe® Kardia equipment in a safe, dry location)
6.
Do not drop or subject MoMe® Kardia parts to extreme physical shock.
7.
The MoMe® Kardia system uses and generates radio frequency energy,
so it may cause harmful interference to radio communication if not used
according to instructions.
8.
The user should take precautions regarding electromagnetic compatibility,
the MoMe® Kardia system needs to be used according to the EMC
information provided in this IFU.
9.
The MoMe® Kardia provided for use in the U.S. will not transmit recorded
data if the patient travels outside of the U.S.
10.
Do not use the MoMe® Kardia system in conditions that are:
— Below 32°F (0°C) or above 104°F (40°C);
— Less than 15% or greater than 93% non-condensing atmospheric humidity.
11.
Do not store or transport MoMe® Kardia in conditions that are:
— Below -25°C or above 70°C;
— Less than 15% or greater than 93% non-condensing atmospheric humidity.

page 14
Battery Information
1. Do not dismantle, open or shred secondary cells or batteries.
2. Do not expose cells or batteries to heat or fire. Avoid storage in direct sunlight.
3. Do not short-circuit a cell or a battery. Do not store cells or batteries
haphazardly in a box or drawer where they may short-circuit each other
or be short-circuited by other metal objects.
4. Do not remove a cell or battery from its original packaging until required
for use.
5. Do not subject cells or batteries to mechanical shock.
6. In the event of a cell leaking, do not allow the liquid to come in contact with
the skin or eyes. If contact has been made, wash the affected area with
copious amounts of water and seek medical advice.
7. Do not use any charger other than that specifically provided for use with
the equipment.
8. Observe the plus (+) and minus (–) marks on the cell, battery and equipment
and ensure correct use.
9. Do not use any cell or battery which is not designed for use with
the equipment.
10.
Battery usage by children should be supervised.
11.
Keep cells and batteries clean and dry.
12. Wipe the cell or battery terminals with a clean dry cloth if they become dirty.
13. Secondary cells and batteries need to be charged before use. Always use
the correct charger and refer to the manufacturer’s instructions or equipment
manual for proper charging instructions.
14. Do not leave a battery on prolonged charge when not in use.
15. After extended periods of storage, it may be necessary to charge and
discharge the cells or batteries several times to obtain maximum performance.
16. Retain the original product literature for future reference.
17. Use only the cell or battery in the application for which it was intended.
18. Dispose of properly.

page 15
System Specifications
MoMe® Kardia Monitor Specification (Model 01854 Rev B)
Specification MoMe® Kardia Monitor
Battery Life Provides 24 hours of function
before recharging
Operating Temperature 5°C to 40°C
Storage Temperature (power off) -25°C to 70°C
Operating Humidity 15% to 93% non-condensing
Storage Humidity 15% to 93% non-condensing
Operating Pressure 700 hPa to 1060 hPa
ECG
Sampling Rate
Digital Resolution
Input Dynamic Range
Input Offset Dynamic Range
200 Hz
5uV
+/- 10 mV
+/- 300 mV
Input Impedence
> 3 MOhm
Peak Current Injection 24 nA (Lead off circuit) DC
RMS Current Injection 29 microA
Data Storage Capacity Minimum 30 days
Dimensions 108 mm x 67 mm x 17 mm max
Weight
80 +/- 5 g
Communication Means LTE, WCDMA
Bands 2,4,5,12,13
Ingress Protection Rating IPX0
Display Type: LED Matrix, Size: 24 X 7
Memory Internal microSD card up to
32 GB, Not user accessible
Battery Li-Ion 1900mAh battery pack, minimum
24 hour battery life

page 16
Electromagnetic Emissions Compliance
The MoMe® Kardia monitor has been tested and found to comply
with the limits for medical devices to the IEC 60601-1-2:2007.
These limits are designed to provide reasonable protection against
harmful interference in a typical installation.
1. This device radiates radio frequency energy in normal use and, if
not installed and used in accordance with instructions in this manual,
may cause harmful interferences to other devices in the vicinity.
If this device does cause harmful interference to other devices,
the user is encouraged to try to correct the interference by one or
more of following measures:
— Reorient or relocate the other device/s
— Increase the separation distances between this device
and other device/s
— Consult the manufacturer/s of other device/s or call
service for help
2. The device performance may be affected by heavy electrical
equipment or other sources of electromagnetic interference.
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
The MoMe® Kardia is intended for use in the electromagnetic environment
specified below. The customer or the user of MoMe® Kardia should assure
that it is used in such an environment
Emissions Test
Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11 Group 1 The MoMe®
Kardia uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11 Class B The MoMe® Kardia is suitable for use in
all establishments, including domestic
establishments and those directly
connected to the public low-voltage power
supply network that supplies buildings used
for domestic purposes.

page 17
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The MoMe® Kardia is intended for use in the electromagnetic environment
specified below. The customer or the user of MoMe® Kardia should assure
that it is used in such an environment
Immunity Test IEC 60601
Test Level Compliance
Level Electromagnetic
Environment - Guidance
Electrostatic
discharge
(ESD) IEC
61000-4-2
±6 kV
contact
±8 kV air
±6 kV
contact
±8 kV air
Floors should be wood, con-
crete or ceramic tile. If floors
are covered with synthetic
material, the relative humidity
should be at least 30%.
Electrical fast
transient/burst +/- 2 kV for
power
supply lines
+/- 1 kV
for input/
output lines
Not
applicable
Surge IEC
61000-4-5 +/- 1 kV line(s)
to line(s)
+/- 2 kV
lines(s)
to earth
Not
applicable
Voltage
dips, short
interruptions
and voltage
variations
on power supply
input lines
IEC 61000-4-11
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5s
Not
applicable
Power
frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical commer-
cial or hospital environment.
page 18
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The MoMe® Kardia is intended for use in the electromagnetic environment
specified below. The customer or the user of MoMe® Kardia should assure
that it is used in such an environment
Immunity
Test
IEC 60601
Test Level
Compliance
Level Electromagnetic
Environment - Guidance
Conducted
RF IEC
61000-4-6
3 Vrms
150 kHz
to 80 MHz
Not
applicable
Portable and mobile RF
communications
equipment should be used no closer to
any part of the
unit, including cables, than
the recommended separation distance
calculated from the equation applicable
to the frequency of the transmitter.
Recommended Separation Distance:
d = 1.2 √ P 80 MHz to 800 MHz
d = 2.3 √ P 800 MHz to 2.5 GHz
where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer
and d is the recommended separation
distance in meters (m). Field strengths
from fixed RF transmitters, as
determined
by an electromagnetic site survey, s
hould
be less than the compliance level in
each
frequency range. Interference may
occur
in the vicinity of equipment marked with
the following symbol:
Radiated
RF IEC
61000-4-3
3 V/m
80 MHz
to 2.5 GHz
Not
applicable
3 V/m
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation
is affected by absorption and reflection from structures, objects and people.
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/
cordless) telephones and land mobile radios, amateur radio, AM and FM radio broad-
cast and TV broadcast cannot be predicted theoretically with accuracy. To assess
the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location in which the
unit is used exceeds the applicable RF compliance level above, then the unit should be
observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as reorienting or relocating the unit.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.

Recommended separation distances between portable and mobile RF
communications equipment and MoMe® Kardia System
The MoMe® Kardia is intended for use in an electromagnetic environment
in which radiated RF disturbances are controlled. The user of the MoMe®
Kardia can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications
equipment (transmitters) and the MoMe® Kardia as recommended below,
according to the maximum output power of the communications equipment
Rated maximum
output power
of transmitter
W
Separation distance according to frequency of transmit
150 kHz
to 80 MHz
d = 1.2 √ P
80 MHz
to 800 MHz
d = 1.2 √ P
800 MHz
to 2,5 GHz
d = 2.3 √ P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1
1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
SAR Exposure Information
This device is designed and manufactured not to exceed the emission limits for exposure
to radio frequency (RF) energy set by the Federal Communications Commission of the
U.S. Government.
The exposure standard employs a unit of measurement known as the Specific Absorption
Rate, or SAR. The SAR limit relevant for the application described in the manual is 1.6W/kg.
Tests for SAR are conducted using standard operating positions accepted by the FCC with
the device transmitting at its highest certified power level in all tested frequency bands.
Although the SAR is determined at the highest certified power level, the actual SAR level
of the equipment while operating can be well below the maximum value. This is because
the device is designed to operate at multiple power levels so as to use only the power
required to reach the network. In general, the closer you are to a wireless base station
antenna, the lower the power output.
Equipment Authorization has been granted to this device with the reported SAR level(s)
evaluated as in compliance with the FCC RF exposure guidelines. SAR information on this
equipment is on file with the FCC and can be found under the Display Grant section of
www.fcc.gov/oet/ea/fccid after searching on the FCC ID as printed on the equipment.
This device has been tested to comply with FCC radiation exposure limits set forth for
an uncontrolled environment when used for the documented intended purpose and when
operated as shown in the user instructions provided with this product, i.e. when carried
with the belt clip coming with the product as a bundled accessory.
The MoMe
®
Kardia Device must be used with the included Belt Clip to keep a safe
minimum distance towards your body (6mm), ensuring compliance with regulatory
limits regarding human exposure to radio frequency radiation.
page 19

FCC Statement:
FCC ID: 2AHLC01855
This device complies with part 15 of the FCC Rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful interference, and (2) this device
must accept any interference received, including interference that may cause undesired
operation.
Changes or modifications not expressly approved by InfoBionic could void the
user's authority to operate the equipment
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which the receiver
is connected.
—Consult the dealer or an experienced radio/TV technician for help
page
20
This manual suits for next models
1
Table of contents
Other InfoBionic Medical Equipment manuals