Bistos BT-450 User manual

Infant Phototherapy Equipment
BT-450
Operation Manual
Please keep this user’s guide for future reference.
P/N: 450-ENG-OPM-EXP-R01

BT-450 Operation manual
P/N:450-ENG-OPM-EXP-R01 Bistos Co., Ltd. 2021.06
2
Table of Contents
Intended use/Indications for use ------------------------------------------------------------------------------3
Product Description --------------------------------------------------------------------------------------3
Contraindications ---------------------------------------------------------------------------------------3
1. Safety Information ------------------------------------------------------------------------------------4
2. Product Configuration --------------------------------------------------------------------------9
2.1 Description of each part --------------------------------------------------------------------9
2.2 Button descriptions ----------------------------------------------------------------------10
2.3 LCD Description -------------------------------------------------------------------------10
3. How to use --------------------------------------------------------------------------------------------------11
3.1 Preparation before use ---------------------------------------------------------------------11
3.2 Patient placement -----------------------------------------------------------------------12
3.3 Product operation ---------------------------------------------------------------------13
3.3.1 Operation mode -------------------------------------------------------------------13
3.3.2 How to operate -------------------------------------------------------------------------13
4. Alarm ---------------------------------------------------------------------------------------------------------15
4.1 Alarm condition --------------------------------------------------------------------------------------15
4.2 Alarm characteristics ------------------------------------------------------------------------------15
5. Precautions for Pad -------------------------------------------------------------------------------16
6. Essential Performance ----------------------------------------------------------------------------16
7. Cleaning and maintenance ----------------------------------------------------------------------16
7.1 Brightness check -------------------------------------------------------------------------16
7.2 Cleaning --------------------------------------------------------------------------------------17
8. Troubleshooting ------------------------------------------------------------------------------------17
9. Manufacturer’s declaration on EMC ------------------------------------------------------------------17
9.1 Electromagnetic emissions ------------------------------------------------------------18
9.2 Recommended separation distances between portable and mobile RF communications
equipment and the Infant Phototherapy Equipment ---------------------------------------18
9.3 Electromagnetic immunity ---------------------------------------------------------------19
10. Product Specifications ---------------------------------------------------------------------------21
Product Warranty ------------------------------------------------------------------------------------------23

BT-450 Operation manual
P/N:450-ENG-OPM-EXP-R01 Bistos Co., Ltd. 2021.06
3
Intended use/Indications for use
The infant phototherapy equipment, BT-450 is indicated for use to treatment of infants diagnosed with
hyperbilirubinemia, commonly known as neonatal jaundice, which can cause a yellow discoloration of the
skin and the whites of the eyes. The devices can be used in a hospital or at home.
The device is designed to use for patient population described in the infant, who is age up to 3 months and
weight less than 10kg.
Product Description
Jaundice refers to the yellow appearance of the skin that
occurs with the deposition of bilirubin in the dermal and
subcutaneous tissue. Bilirubin is the orange-yellow pigment
of bile, formed principally by the breakdown of hemoglobin
in red blood cells at the end of their normal lifespan.
Normally in the body, bilirubin is processed through the liver,
where it is conjugated to glucuronic acid by the enzyme in
the liver. This conjugated form of bilirubin is then excreted
into the bile and removed from the body via the gut. When
this excretion process is low following birth, does not work
efficiently, or is overwhelmed by the amount of
endogenously produced bilirubin, the amount of bilirubin in the body increases, resulting in
hyperbilirubinemia and jaundice.
In newborns, the lifespan of red blood cells is shorter than that of adults, which makes a lot of bilirubin, the
function of an enzyme to conjugate the bilirubin is poor, and the function to excrete bilirubin out of the
body is also weak.
Phototherapy refers to the use of light to convert unconjugated bilirubin molecules into water-soluble
isomers that can be excreted in bile or urine without the need for conjugation. Bilirubin absorbs light most
strongly in the blue region of the spectrum near 460 nm, a region in which penetration of tissue by light
increases markedly with increasing wavelength. Only wavelengths that penetrate tissue and are absorbed
by bilirubin have a phototherapeutic effect. Lamps with output predominantly in the 460-to-490-nm blue
region of the spectrum are probably the most effective for treating hyperbilirubinemia.
The infant phototherapy equipment, BT-450 pad consists of LEDs that emits light of peak wavelength 455
to 465 nm. The microcontroller generates the PWM, and it is rectified to direct current through a resistor
and a capacitor. When the rectified PWM is output through the LED driver, the LED can be stably turned on.
The intensity of light can be adjusted by changing the duty cycle of PWM, and BT-450 has two types of
intensity, high and low.
WARNING
Eye Protection: Do not look directly into the LED. During the treatment, always use
a patch or equipment to protect a baby’s eyes.
Periodically, check the hospital or treatment protocol and makes sure that the
baby’s eyes are protected from contamination.
Patients near the light should use protective pads or equipment to protect their
eyes.
Contraindications
It should not be used in cases of congenital porphyria, a family history of porphyria, and treatment with
photosensitive drugs or medicines.

BT-450 Operation manual
P/N:450-ENG-OPM-EXP-R01 Bistos Co., Ltd. 2021.06
4
1. Safety information
Before using the infant phototherapy equipment, read this entire manual and be fully understood, and
follow instructions and safety information to prevent injury.
Symbols and safety sign used:
The following symbols and safety signs identify all instructions that are important to safety. Failure to
follow these instructions can lead to injury or damage to the infant phototherapy equipment. When used
in conjunction with the following words, the symbols indicate:
WARNING
Can incur serious injury or death.
CAUTION
Can incur minor injury or product/property damage
The following symbols and safety signs are placed on the product, label, packing, and this manual to stand
for the information about:
Symbol
Standard/Symbol Reference no.
Description
ISO 7010 —Graphical symbols —Safety
colours and safety signs —Registered
safety signs / W001
Used to display safety information for
warnings.
Before using the BT-450, please be fully
understand the information provided with
the device.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.4.4
Used to display safety information for
caution.
Before using the BT-450, please be fully
understand the information provided with
the device.
IP21
IP22
IP23
IEC 60529 Degrees of protection
provided by enclosures
These indicate the protection level against
the ingress of solid objects and liquid.
IPX1 is protection against falling water
drops vertically.
IPX2 is protection against some falling
water drops vertically when the enclosure
tilted up to 15°.
IPX3 is protection against spraying water
at any angle up to 60° from the vertical
shall.
IP2X is protection against solid foreign
objects like a finger.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.4.3
Refer to the operation manual. Read the
manual before placing the device.
ISO 7010 —Graphical symbols —Safety
colours and safety signs —Registered
safety signs / M002
Refer to the operation manual. Read the
manual before placing the device.
IEC 60417 —Graphical Symbols for Use
on Equipment / 5032
This symbol means alternating current.
IEC 60417 —Graphical Symbols for Use
on Equipment / 5031
This symbol means a DC power adapter.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
This symbol indicates the manufacturer.

BT-450 Operation manual
P/N:450-ENG-OPM-EXP-R01 Bistos Co., Ltd. 2021.06
5
Part 1: General requirements / 5.1.1
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.1.3
This symbol indicates the date of
manufacture.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.1.7
This symbol indicates the serial number of
the device.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.1.6
This symbol indicates a reference number.
IEC 60417 —Graphical Symbols for Use
on Equipment / 5172
This symbol means the power adapter is a
Class II device.
IEC 60417 —Graphical Symbols for Use
on Equipment / 5333
This symbol indicates the BF applied part.
This applies to the Pad.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.4
This symbol indicates to keep the device
dry.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.1
This symbol indicates the medical device
that can be broken or damaged if not
handled carefully.
ISO 7000 —Graphical symbols for use on
equipment -- Registered symbols / 0623
This symbol indicates to keep upright
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.2
This symbol indicates to keep the device
away from sunlight.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.7
This symbol indicates the temperature
limitation for transport and storage.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.8
This symbol indicates the humidity
limitation for transport and storage.
ISO 15223-1, Medical Devices—Symbols
to be used with medical device labels,
labeling and information to be supplied –
Part 1: General requirements / 5.3.9
This symbol indicates the range of
atmospheric pressure to which the medical
device can be safely exposed for transport
and storage.
Universal Recycling symbol
This symbol indicates the packing material
is recyclable.
IEC TR 60878, Graphical symbols for
electrical equipment in medical practice
This symbol indicates that always protect
the infant’s eyes with eye patches or
equivalent.
Table of contents
Other Bistos Medical Equipment manuals
Bistos
Bistos BT-550 User manual
Bistos
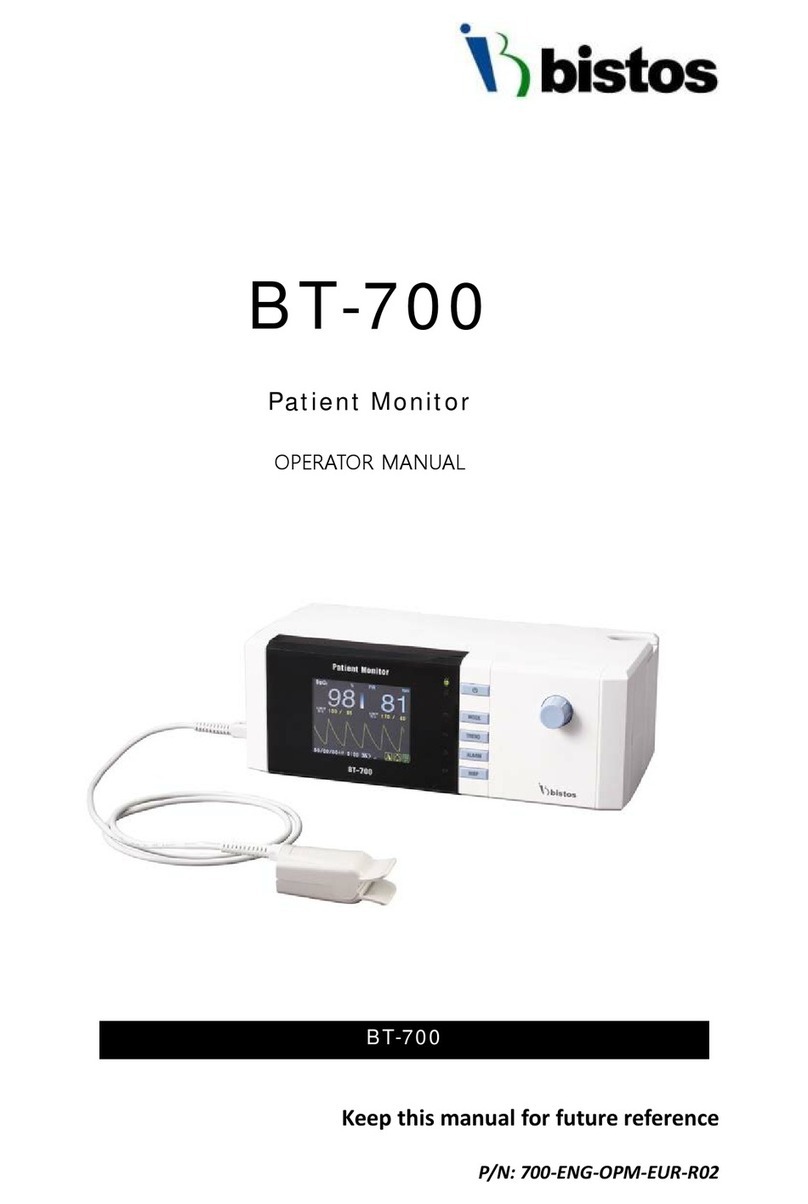
Bistos BT-700 User manual
Bistos
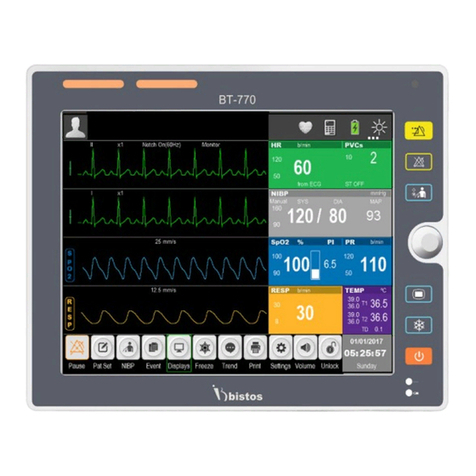
Bistos BT-770 User manual

Bistos
Bistos BT-750 User manual

Bistos
Bistos BT-500 User manual

Bistos
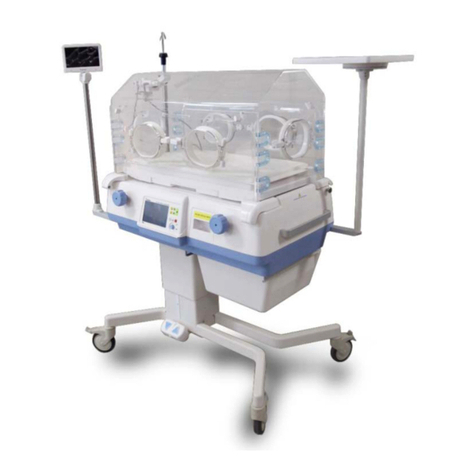
Bistos BT-720 User manual
Bistos
Bistos BT-500 User manual

Bistos
Bistos BT-400 User manual
Bistos
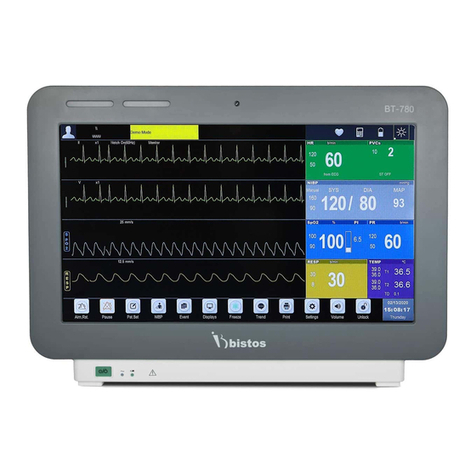
Bistos BT-780 User manual

Bistos
Bistos BT-250 User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual