inofab Spirohome Clinic 02000 User manual

spirohome
®
│Clinic
User Manual
Welcome to Spirohome®
Before using your Spirohome® Clinic device and mobile application, please ensure
that you have read this user manual and labels and information on the product. This
user manual is for these models: Spirohome® Clinic 02000, Spirohome® Clinic
02010, Spirohome® Clinic 02020. Inofab can provide user manual physical print or
digital (from İnofab Apps and İnofab websites).

spirohome
®
│Clinic
User Manual
1 / 33
1. CONTENTS
CONTENTS 1
INTRODUCTION 2
PRODUCT DESCRIPTION 2
WHAT’S IN THE BOX 2
INTENDED USE 2
RESTRICTIONS ON USE AND CONTRAINDICATIONS 3
PARAMETERS 4
OPERATION 7
OPERATING ENVIRONMENT 7
SETTING UP YOUR DEVICE 7
DEVICE INDICATORS 8
PERFORMING A LUNG FUNCTION TEST 9
General Method For Performing a Spirometry Test with The Spirohome® Clinic: 10
Types of Breathing Maneuvers 12
End of the Tests 16
UNDERSTANDING THE TEST QUALITY 17
SIGNS AND SYMBOLS 19
TECHNICAL FEATURES 20
SAFETY WARNINGS AND PRECAUTIONS 20
MAINTENANCE 22
CALIBRATION-CHECK 23
Preparation of Calibration Check 23
CLEANING AND DISINFECTION 24
THE SPIROWAY DISPOSABLE 25
BATTERIES 25
DISPOSAL OF SPIROHOME 26
TROUBLESHOOTING 27
ORDERABLE ACCESSORIES 29
TERMS OF WARRANTY 29
ELECTROMAGNETIC COMPATIBILITY 30
MANUFACTURER INFORMATION 34
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
2 / 33
2. INTRODUCTION
2.1. PRODUCT DESCRIPTION
The Spirohome® Clinic is a portable spirometer that pairs (via Bluetooth®
) and operates with
smart devices running with iOS, Android, or Windows. The Spirohome® Clinic measures and
displays certain parameters of the lung function of the user. The user performs a spirometry
test as described in the Performing A Lung Function Test section of this user manual. Briefly,
as the user exhales into the device through its mouthpiece, internal ultrasonic sensors detect
the velocity of the expired air, the device converts this information into spirometric data and
displays it via the Spirohome® application. The Spirohome® app prompts and guides the user
throughout the test for accurate data collection and registration. The app can be downloaded
on Apple’s App Store, Google Play Store, Microsoft Store. The device is powered by 2 x
AAA batteries. The Spirohome®
Clinic works with the Spiroway Disposable mouthpiece.
2.2. WHAT’S IN THE BOX
Your Spirohome®
Clinic box contains:
· Spirohome®
Clinic Device
· Spirohome®
Clinic Cap
· Quick start guide
· Calibration Certificate
⚠ Caution: Please check to ensure that there is no visible damage on any of the
components of the Spirohome® Clinic. If the damage is present, do not use or attempt to
repair the device, please contact the manufacturer directly.
2.3. INTENDED USE
The Spirohome® Clinic is intended to be used as a portable spirometer used in lung function
testing for several parameters. See Parameters section for more information about
measured parameters. The Spirohome®
Clinic is indicated for:
- children (over the age of 5), adolescents or adults who have been diagnosed with a
chronic pulmonary disease including, but not limited to, asthma, chronic obstructive
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
3 / 33
pulmonary disease and cystic fibrosis. These measurements can be used for the
detection, assessment and monitoring of diseases affecting the lung function.
and should be used by:
- Healthcare professionals, test operators, physicians, clinicians, occupational health
professionals, etc.
2.4. RESTRICTIONS ON USE AND CONTRAINDICATIONS
Any diagnosis of conditions or prescribed treatments should be made only by a qualified
healthcare professional who, in addition to the test results provided by Spirohome® Clinic,
will take into consideration the outcomes of a medical examination, the patient’s clinical
history and results of any other tests deemed necessary.
Spirohome® Clinic is a multi-user device. The device can log the information and test results
that belong to each specific patient. For each new patient, a new patient account must be
created on the Spirohome® Clinic app, so that each user's personal information and test
results can be stored and logged.
A new Spiroway Disposable mouthpiece must be used for each new user.
The spirometry test should only be performed by users who do not experience any shortness
of breath and are in good health for performing a lung function test. Test results of patients
who do not meet these conditions may not be reliable. A correct spirometry test depends
greatly on the patient’s ability to correctly perform the expiratory/inspiratory maneuver as
described in this manual. Failure to perform a correct maneuver may lead to inaccurate and
unacceptable results. The device should not be used if the accuracy and reliability of test
results may be jeopardized by external factors.
Performing spirometry can be physically demanding. The forced expiratory maneuver used
in spirometry increases intrathoracic, intraabdominal, and intracranial pressures. Potential
risks of spirometry are primarily related to maximal pressures generated in the thorax and
their impact on abdominal and thoracic organs, venous return and systemic blood pressure,
and expansion of the chest wall and lung. The physical effort required can increase
myocardial demand. Caution must be used for patients with medical conditions that could be
adversely affected by these physiological consequences. Although such risks are likely to be
minimal for spirometry in most patients, the potential risks associated with testing should
always be weighed against the benefit of obtaining information about lung function.
Spirometry should be discontinued if the patient experiences pain during the maneuver.
Patients with potential contraindications that would prevent testing in the primary care setting
may be tested in a pulmonary function laboratory where operators are more experienced
and there may be access to emergency care if needed. Furthermore, because spirometry
requires the active participation of the patient, the inability to understand directions or
unwillingness to follow the directions of the operator will usually lead to submaximal test
results.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
4 / 33
Relative Contraindications for Spirometry;
Due to increases in myocardial demand or changes in blood pressure;
➢Acute myocardial infarction within 1 wk
➢Systemic hypotension or severe hypertension
➢Significant atrial/ventricular arrhythmia
➢Noncompensated heart failure
➢Uncontrolled pulmonary hypertension
➢Acute cor pulmonale
➢Clinically unstable pulmonary embolism
➢History of syncope related to forced expiration/cough
Due to increases in intracranial/intraocular pressure;
➢Cerebral aneurysm
➢Brain surgery within 4 wk
➢Recent concussion with continuing symptoms
➢Eye surgery within 1 wk
Due to increases in sinus and middle ear pressures;
➢Sinus surgery or middle ear surgery or infection within 1 wk
Due to increases in intrathoracic and intraabdominal pressure;
➢Presence of pneumothorax
➢Thoracic surgery within 4 wk
➢Abdominal surgery within 4 wk
➢Late-term pregnancy
Infection control issues;
➢Active or suspected transmissible respiratory or systemic infection, including
tuberculosis
➢Physical conditions predisposing to the transmission of infections, such as
hemoptysis, significant secretions, or oral lesions or oral bleeding
Please ask the patient if they have or suspect having any of the conditions above before the
use of the Spirohome®
Clinic.
2.5. PARAMETERS
The Spirohome®
Clinic records and displays the following spirometry data:
Parameters
Definition
Unit
FVC
Forced Vital Capacity — The volume of air that can forcibly be
blown out after full inspiration
L
FEV0.75
Forced Expiratory Volume within 0.75 seconds: The volume of air
that can forcibly be blown out within 0.75 seconds, after full
inspiration.
L
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
5 / 33
FEV1
Forced Expiratory Volume within 1 second
L
FEV3
Forced Expiratory Volume within 3 seconds
L
FEV6
Forced Expiratory Volume within 6 seconds
L
FEV0.75
/FVC
The ratio of FEV0.75
to FVC
--
FEV1
/FVC
The ratio of FEV1
to FVC
--
FEV3
/FVC
The ratio of FEV3
to FVC
--
FEV6
/FVC
The ratio of FEV6
to FVC
--
PEF
Peak Expiratory Flow — The maximal flow rate achieved during
the maximally forced expiration initiated at full inspiration.
L/s
MMEF
Mean Mid-Expiratory Flow — synonymous with FEF25-75
L/s
FEF25
Forced Expiratory Flow at 25% of vital capacity — synonymous
with MEF75
L/s
FEF50
Forced Expiratory Flow at 50% of vital capacity — synonymous
with MEF50
L/s
FEF75
Forced Expiratory Flow at 75% of vital capacity —synonymous
with MEF25
L/s
FEF25-75
Forced Expiratory Flow from 25% to 75% of vital capacity —
synonymous with MMEF
L/s
MET25-75
Mid-Expiratory Time — synonymous with FET
25-75
s
FEV0.75
/FEV6
The ratio of FEV0.75
to FEV6
--
FEV1
/FEV6
The ratio of FEV1
to FEV6
--
FEF50
/FVC
The ratio of FEF50
to FVC
1/s
MMEF/FVC
The ratio of MMEF to FVC
1/s
FET
Forced Expiratory Time
s
BEV
Back extrapolated volume
L
FIV1
The forced inspiratory volume within 1 second
L
FIVC
Forced inspiratory vital capacity
L
PIF
Peak inspiratory flow
L/s
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
6 / 33
FIF25-75
Forced inspiratory flow at 25% of vital capacity — synonymous
with MIF75
L/s
FIV1
/FIVC
The ratio of FIV1
to FIVC
--
R50
(FEF50
/FIF50
)
The ratio of flow at 50% of expiration and flow at 50% of
inspiration — synonymous with FEF50
/FIF50
--
VC
Vital capacity, from slow expiration
L
VCin
Inspiratory vital capacity, from slow inspiration
L
VCex
Expiratory vital capacity, from slow expiration
L
ERV
Expiratory reserve volume
L
IRV
Inspiratory reserve volume
L
IC
Inspiratory capacity from end of tidal breathing
L
Rf
Respiratory frequency
1/min
VT
Tidal Volume
L
MVV
Maximum voluntary ventilation
L/min
MVV6
Maximum plat voluntary ventilation for 6 seconds
L/min
MVVtime
Duration of the trial in seconds
s
The recommended number of trials per spirometry session is 3, however, the user may
perform up to 8 trials. The best values obtained from the spirometry tests performed in one
session are displayed on the app interface. Users and healthcare professionals have the
option to view the results of each spirometry trial performed in a spirometry session
separately.
The device also provides a reference value (obtained from large epidemiological studies)
based on the patient’s height, weight, age, sex and ethnicity. Test results from spirometry
tests are compared to the reference value and displayed as a percent predictive value
indicator of the patient’s respiratory health. The patient’s personal best value for a spirometry
session should be discussed with them for medical interpretation.
⚠ Caution: Interpretation of spirometry results or diagnosis of medical conditions, if any, is
to be made by a physician or allied health care professional with sufficient training in the
performance and interpretation of spirometry.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
7 / 33
3. OPERATION
3.1. OPERATING ENVIRONMENT
The Spirohome®
Clinic is designed for use in a clinical setting, by more than one user.
The operating conditions for the Spirohome®
Clinic are specified as:
Temperature: +15°C to +35°C
Relative Humidity: 10% to 85%
The storage conditions for the Spirohome®
Clinic are specified as:
Temperature: -20°C to +60°C
Relative Humidity: 0% to 85%
Pressure: 500 hPa to 1060 hPa
The Spirohome® Clinic should not be used in the presence of flammable liquids or
detergents, nor in the presence of inflammable anesthetic gases (oxygen or nitrogen). The
device should not be used in direct air currents (e.g. wind), sources of heat or cold, direct
sun rays or other sources of light or energy, dust, sand or any other chemical substances.
3.2. SETTING UP YOUR DEVICE
1. Download the Spirohome® Clinic app from the App Store, Google Play Store, or
Microsoft Store into a smart device or PC.
2. Follow the steps given in the app to create an account for a new user or login to an
existing account.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
8 / 33
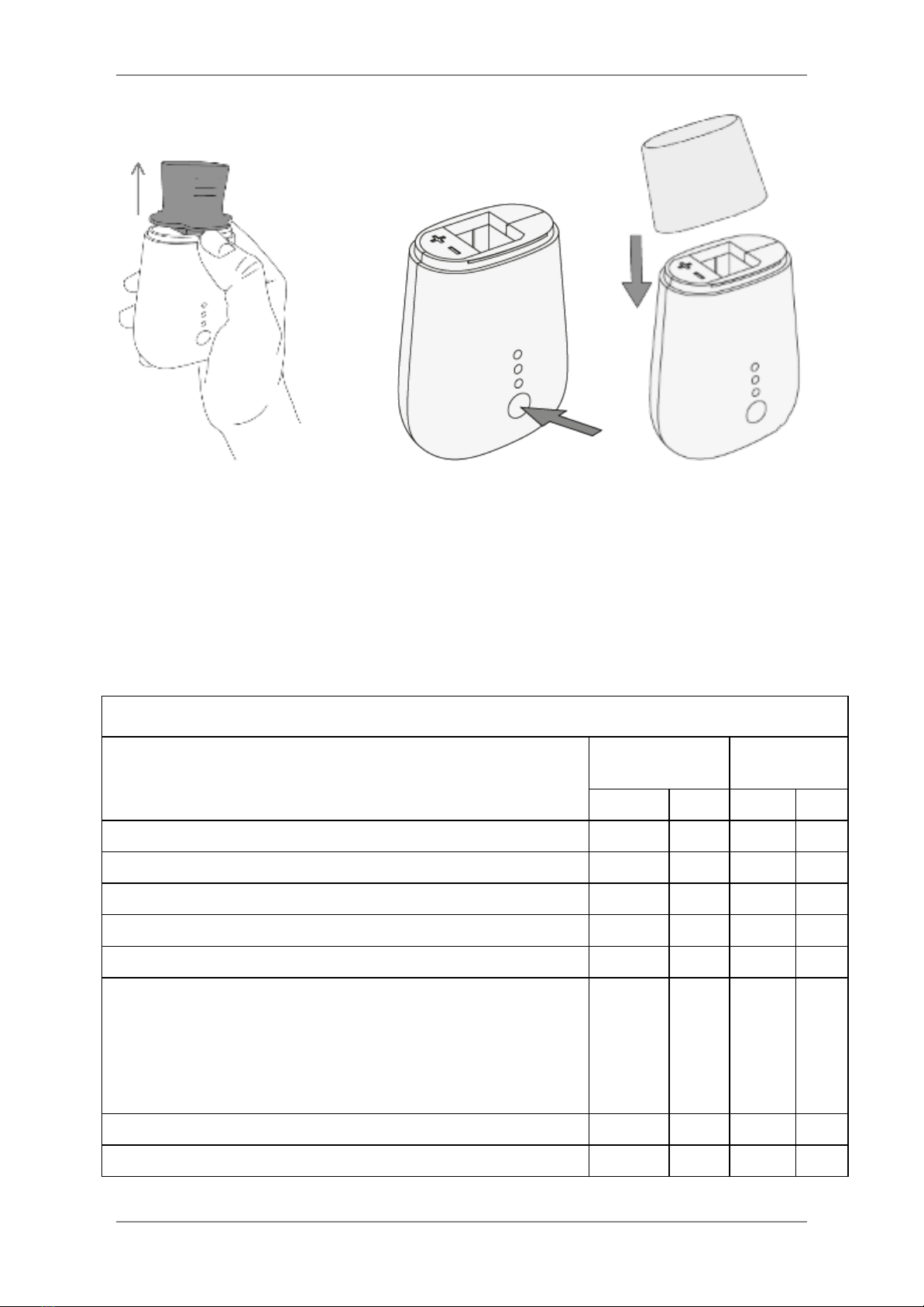
3. Remove the battery cover by sliding it,
place the batteries in the correct
orientation, slide the battery cover back to
the closed position and press on the power
button to switch the device on as shown.
4. Enable Bluetooth on the smart device or PC and pair the Spirohome® Clinic with the
smart device or PC, following the instructions on the app.
3.3. DEVICE INDICATORS
There are 3 LED lights located on the front of
the device. The LED lights may be turned on
or flashing various colors and/or in various
patterns. The LED lights indicate the current
status of the device. Please see the following
information for guidance on LED light
indications.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
9 / 33
LED Display
Indication/s
None of the LEDs is on.
The device is switched off.
LED indicators are consecutively flashing
green.
The device is switching on.
LED number 3 is a constant green.
The device is switched on.
LED number 2 is fading on and off in blue.
The device is connected to the app. Bluetooth®
connection has been established.
LED number 2 and LEDs 1 and 3 together are
flashing yellow in turn.
The zero flow level adjustment is setting up.
LED number 1 is a constant blue.
The device is ready for a test.
During a test, LED number 1 is constant yellow.
The test has timed-out (there has been no
inhalation/exhalation over a period of time)
During zero flow level adjustment LED number
1 is constant yellow.
The zero flow level adjustment has been
unsuccessful.
All LEDs are flashing red.
There is a foreign object between the sensors.
(Check device error in troubleshooting section)
LEDs are consecutively flashing yellow.
Over-the-air connection is being established.
LED number 3 flashes red three times.
Battery low warning.
LEDs flash in reverse order and remain
switched off.
The device is switching off.
3.4. PERFORMING A LUNG FUNCTION TEST
There are several types of tests and different parameters related to lung function that can be
involved in a spirometry test. Each type of spirometry test requires a specific breathing
maneuver to detect the parameters related to that particular test type. However, the general
method of performing a spirometry test remains the same for all test types. Please keep
reading for more information about test types, test parameters, breathing maneuvers and
understanding the quality of test results.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
10 / 33
3.4.1. General Method For Performing a Spirometry Test with The
Spirohome
®
Clinic:
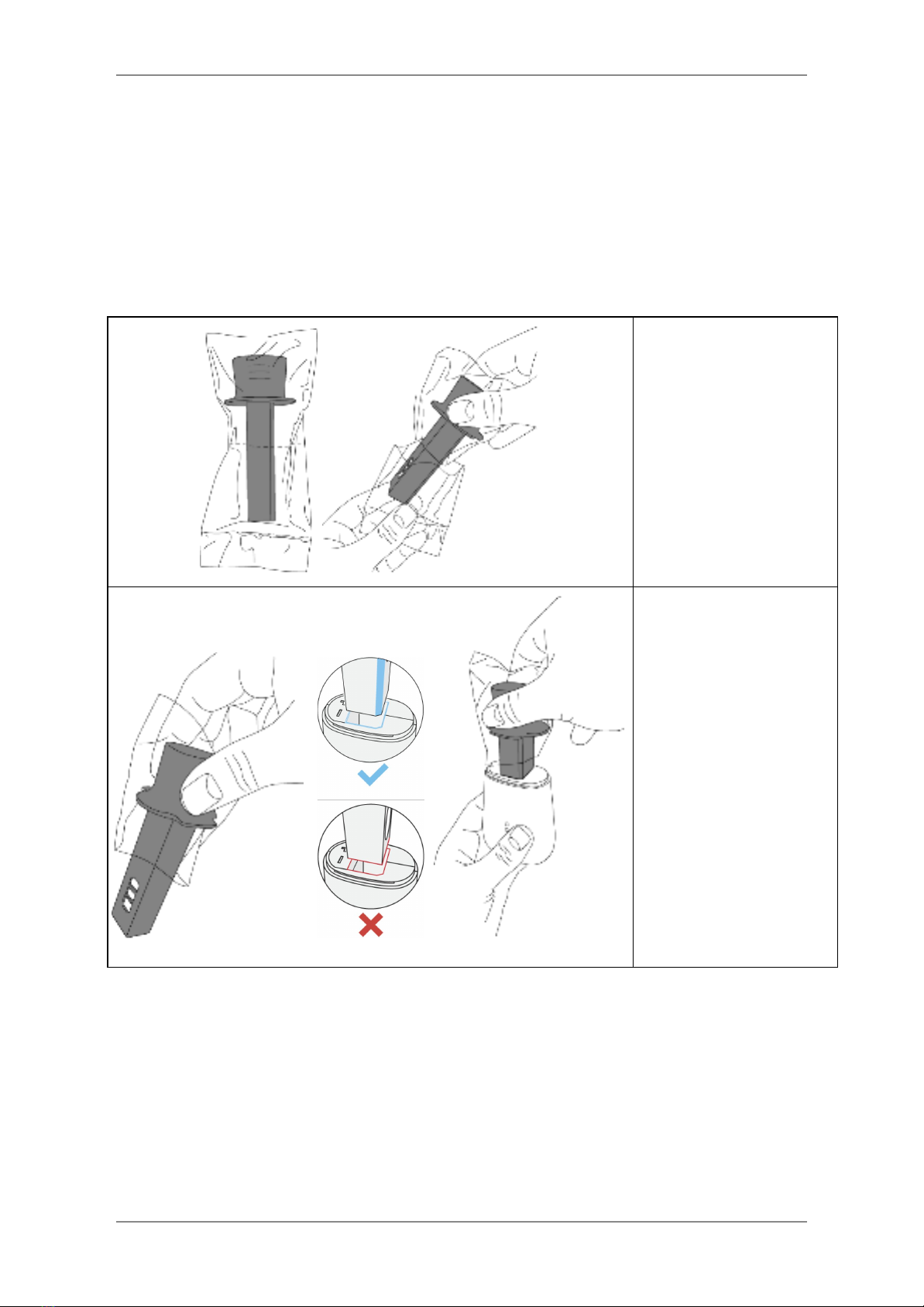
1. Open the cap of the Spirohome®
Clinic device.
2. Remove the Spiroway Disposable mouthpiece from its plastic packaging and insert it
all the way into the Spirohome®
Clinic in the correct orientation (as shown).
Tear the bottom part of
the mouthpiece’s plastic
package and do not
touch the mouthpiece
with bare hands.
Hold the mouthpiece
with the upper part of
the plastic package.
Insert the mouthpiece all
the way into the
Spirohome® Clinic
device in the correct
orientation.
3. A ‘click’ will be heard when the mouthpiece is inserted correctly all the way into the
device.
4. Open the Spirohome® App on your smart device or PC. If you are not registered,
register to the Spirohome® app by creating a new user account and then log in. If the
patient is not registered, enter the patient information from the new patient section
and register the patient.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
11 / 33
5. Select the patient name from the patient list and tap the plus button on the screen to
start the test procedure from the patient details page.
6. The first steps will be to select test mode and enter the ambient conditions like
temperature and relative humidity (in some test modes) and then adjust the zero flow
level for the device. To perform zero flow level adjustment stabilize the device during
the zero flow level adjustment process. Alternatively, place the device on a flat
surface and allow the zero flow level adjustment to be completed. Make sure that
there is no airflow around the device during the zero flow level adjustment process.
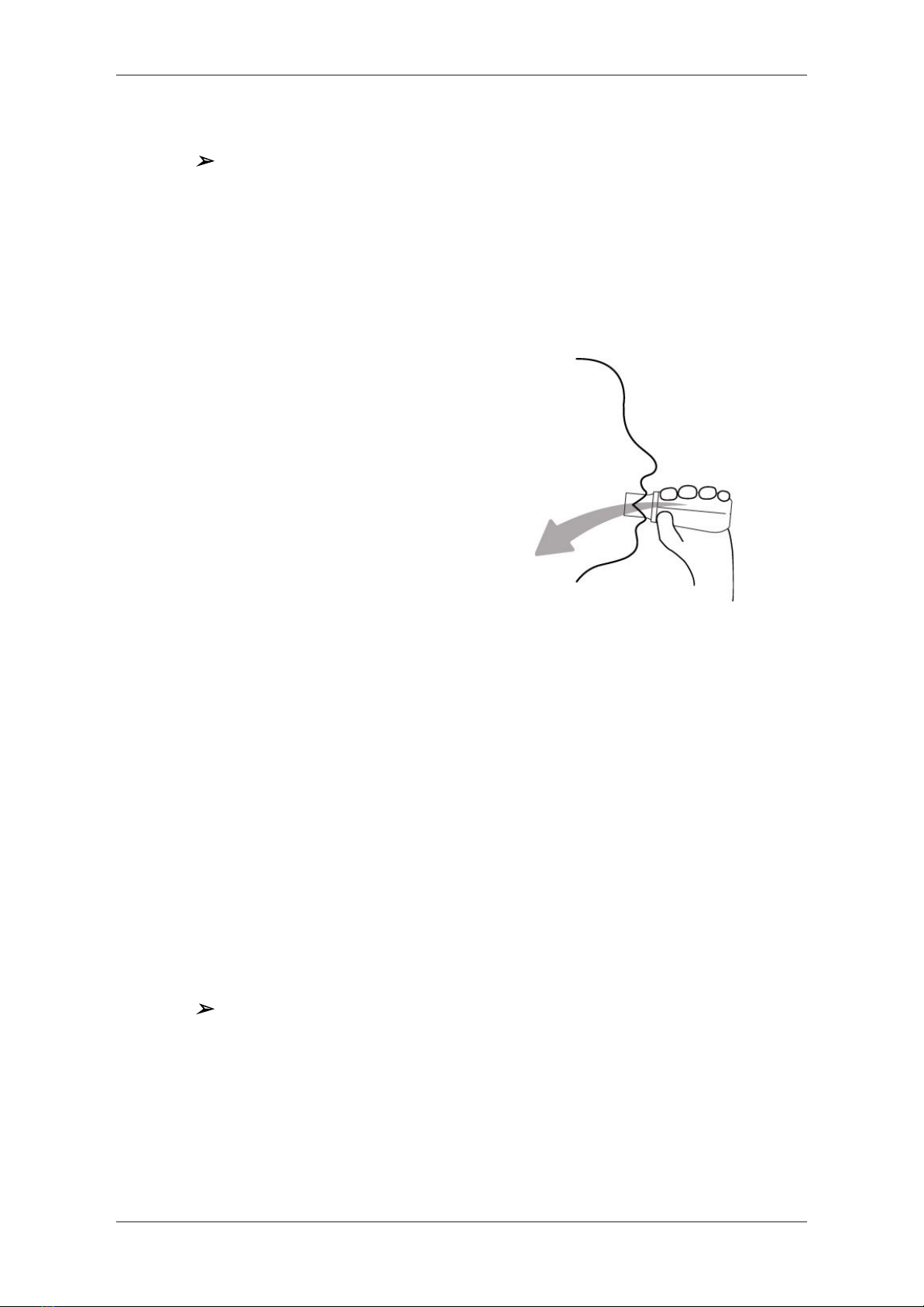
7. The app will prompt the operator to
start a spirometry test. Let the patient
sit with his back straight and his feet
resting on the ground. The patient
must place the mouthpiece in their
mouth, past their teeth (necessary
for measurement accuracy) and form
a tight seal around the mouthpiece
with their lips.
8. The patient should now perform the breathing maneuver related to the particular
spirometry test. Please continue to the Type of Breathing Maneuvers section for
more information.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
12 / 33
3.4.2. Types of Breathing Maneuvers
➢
The Forced Vital Capacity (FVC) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the FVC test mode and the test screen
will appear.
2. Then adjust the zero flow level for the device.
3. The patient will now need to perform a forced expiratory maneuver. To get ready, the
patient should inhale and exhale normally a couple of times.
4. Ask the patient to place the
mouthpiece in his/her mouth, past
his/her teeth and ensure that his/her
lips are tightly sealed around the
mouthpiece, then the patient takes a
fast and deep breath, filling his/her
lungs as much as possible. The
breath taken should not be kept for
more than 2 seconds.
5. Keeping his/her lips sealed tightly around the mouthpiece, the patient must blow out
the inhaled air and empty his/her lungs as hard and fast as the patient can into the
mouthpiece and keep blowing until completely emptying his/her lungs without
breaking the seal of his/her lips.
6. If it takes more than 15 seconds to empty all the air from his/her lungs with the right
performance, the test will be completed automatically. The patient may use a nose
clip to help him/her to exhale only through his/her mouth during the forced exhalation
maneuver.
7. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver has been completed.
8. The test results will be displayed on the app screen. Give feedback to the patient on
his/her effort by looking at the test results. The patient will need to perform 2 more
tests by repeating this breathing maneuver. However, please make sure that the
patient has time to rest between tests and feels well enough to continue.
➢
Tidal Forced Vital Capacity (Tidal FVC) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the Tidal FVC test mode and the test
screen will appear.
2. Enter the required ambient conditions (makes sure you entered the correct values as
the measurement may be significantly affected by a wrong value) like temperature
and relative humidity and then adjust zero flow level for the device.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
13 / 33
3. Ask the patient to place the mouthpiece in his/her mouth, past his/her teeth and
ensure that his/her lips are tightly sealed around the mouthpiece.
4. To get ready, the patient should inhale and exhale normally at least 3 times, then
take a fast and deep breath, filling your lungs as much as possible. The breath taken
should not be kept for more than 2 seconds.
5. Keeping his/her lips sealed tightly around the mouthpiece, the patient must blow out
this inhaled air and empty his/her lungs as hard and fast as the patient can into the
mouthpiece and keep blowing until completely emptying his/her lungs without
breaking the seal of his/her lips.
6. The patient may use a nose clip to help him/her to exhale only through his/her mouth
during the breath maneuver.
7. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver has been completed.
8. The test results will be displayed on the app screen. Give feedback to the patient on
his/her effort by looking at the test results. The patient will need to perform 2 more
tests by repeating this breathing maneuver. However, please make sure that the
patient has time to rest between tests and feels well enough to continue.
NOTE: In the Tidal FVC mode the patient breathes normally into the device a few times at
the beginning of the test and data is recorded as soon as a forced expiration is detected,
whereas in FVC test mode the test is initiated directly with a forced expiratory maneuver.
➢The Flow Volume Loop (FVL) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the FVL test mode and the test screen
will appear.
2. Enter the required ambient conditions (makes sure you entered the correct values as
the measurement may be significantly affected by a wrong value) like temperature
and relative humidity and then adjust zero flow level for the device. To get ready, the
patient should inhale and exhale normally a couple of times.
3. Ask the patient to place the mouthpiece in his/her mouth, past his/her teeth and
ensure that his/her lips are tightly sealed around the mouthpiece, then take a slow
and deep breath, filling his/her lungs as much as possible.
4. Keeping his/her lips sealed tightly around the mouthpiece, the patient must blow out
this inhaled air and empty his/her lungs as hard and fast as the patient can into the
mouthpiece and keep blowing for at least 6 seconds without breaking the seal of
his/her lips.
5. After the patient exhales whole air from the lungs, without breaking the seal of his/her
lips, the patient must inhale completely to fill his/her lungs. When performing this
breathing maneuver, the patient must make sure to keep blowing until the patient has
completely emptied his/her lungs. The patient may use a nose clip to help him/her to
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
14 / 33
inhale and exhale only through his/her mouth during this breathing maneuver.
6. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver has been completed.
7. The test results will be displayed on the app screen. Give feedback to the patient on
his/her effort by looking at the test results. The patient will need to perform 2 more
tests by repeating this breathing maneuver. However, please make sure that the
patient has time to rest between tests and feels well enough to continue.
➢
Tidal Flow Volume Loop (Tidal FVL) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the Tidal FVL test mode and the test
screen will appear.
2. Enter the required ambient conditions (makes sure you entered the correct values as
the measurement may be significantly affected by a wrong value) like temperature
and relative humidity and then adjust zero flow level for the device.
3. Ask the patient to place the mouthpiece in his/her mouth, past his/her teeth and
ensure that his/her lips are tightly sealed around the mouthpiece.
4. To get ready, the patient inhales and exhales normally at least 3 times, then takes a
slow and deep breath, filling his/her lungs as much as possible.
5. Keeping his/her lips sealed tightly around the mouthpiece, the patient must blow out
this inhaled air and empty his/her lungs as hard and fast as the patient can into the
mouthpiece.
6. After the patient exhales whole air from the lungs, without breaking the seal of his/her
lips, the patient must inhale completely to fill his/her lungs. When performing this
breathing maneuver, the patient must make sure to keep blowing until the patient has
completely emptied his/her lungs. The patient may use a nose clip to help him/her to
inhale and exhale only through his/her mouth during this breathing maneuver.
7. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver has been completed.
8. The test results will be displayed on the app screen. Give feedback to the patient on
his/her effort by looking at the test results. The patient will need to perform 2 more
tests by repeating this breathing maneuver. However, please make sure that the
patient has time to rest between tests and feels well enough to continue.
NOTE: In the Tidal FVL mode the patient breathes normally into the device a few times at
the beginning of the test and data is recorded as soon as a forced expiration is detected,
whereas in FVL test mode the test is initiated directly with a forced expiratory maneuver.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
15 / 33
➢End of Forced Expiration (EOFE)
Some standards stress the importance of a maximal inspiration after the forced expiration.
As such, the end of forced expiration (EOFE) is not the end of the maneuver, and hence the
term EOFE is used.
Recognizing a satisfactory EOFE is important to ensure that a true FVC has been achieved.
Achieving one of the following three recommended indicators of EOFE is required:
1. There is less than a 0.025 L change in volume for at least 1 second (a “plateau”).
OR
2. The patient has achieved an FET of 15 seconds.
OR
3. FVC is within the repeatability tolerance of or is greater than the largest prior
observed FVC *
* Occurs when the patient cannot expire long enough to achieve a plateau (e.g., children
with high elastic recoil or patients with restrictive lung disease) or when the patient inspires
or comes off the mouthpiece before a plateau. For within-maneuver acceptability, the FVC
must be greater than or within the repeatability tolerance of the largest FVC observed before
this maneuver within the current prebronchodilator or the current post-bronchodilator testing
set.
➢
The Maximum Voluntary Ventilation (MVV) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the MVV test mode and the test screen
will appear.
2. Enter the required ambient conditions (makes sure you entered the correct values as
the measurement may be significantly affected by a wrong value) like temperature
and relative humidity and then adjust zero flow level for the device.
3. Ask the patient to place the mouthpiece in his/her mouth, past his/her teeth and
ensure that the patient's lips are tightly sealed around the mouthpiece.
4. When the test starts, the patients should inhale and exhale normally at least 4 times,
then inhale and exhale completely filling and emptying their lungs, repeatedly,
uninterrupted, deeply, without breaking the seal of their lips for at least 12 seconds.
The patient may use a nose clip to help him/her to inhale and exhale only through
his/her mouth during this breathing maneuver.
5. Actively encourage the patient to breathe deeply and rapidly moving as much air as
possible for at least 12 seconds.
6. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver has been completed.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020

spirohome
®
│Clinic
User Manual
16 / 33
7. The test results will be displayed on the app screen. If the test fails, give feedback
and guide the patient for another trial. Encourage them to breathe deep and fast and
try to reach at least 12 seconds.
➢
The Slow Vital Capacity (SVC) Test Breathing Maneuver:
1. Ensure that the device is connected. Select the SVC test mode and the test screen
will appear.
2. Enter the required ambient conditions (makes sure you entered the correct values as
the measurement may be significantly affected by a wrong value) like temperature
and relative humidity and then adjust zero flow level for the device.
3. Tell the patient to wear a nose clip and ask the patient to place the mouthpiece in
his/her mouth, past his/her teeth and ensure that his/her lips are tightly sealed
around the mouthpiece.
4. When the test starts, the patient should inhale and exhale normally at least 4 times,
then the patient should inhale as deep as the patient can and fill his/her lungs
completely.
5. After that, the patient should exhale the whole air in his/her lungs gently and slowly
until the patient feels that all the air in his/her lungs feels completely empty without
breaking the seal of his/her lips.
6. When performing this breathing maneuver, the patient must make sure to keep
blowing until the patient feels like the patient has completely emptied his/her lungs.
7. The test can also be performed by performing the breath maneuver in the opposite
direction. When the test starts, the patient should inhale and exhale normally at least
4 times, then the patient should exhale as deep as the patient can and empty his/her
lungs completely. After that, the patient should inhale all the air in his/her lungs until
s/he feels completely full without breaking the seal of his/her lips.
8. The patient may remove the mouthpiece from his/her mouth and resume normal
breathing once the breathing maneuver is complete.
9. The test results will be displayed on the app screen. Give feedback to the patient on
his/her effort by looking at the test results. The patient will need to perform 2 more
tests by repeating this breathing maneuver. However, please make sure that the
patient has time to rest between tests and feels well enough to continue.
3.4.3. End of the Tests
Once all tests have been satisfactorily completed, you will be able to view the session results
on the results page of the app. After the end of the spirometry session, remove and
immediately dispose of the mouthpiece by pushing the notch without touching the top part of
the mouthpiece. Turn the device off by pressing the power button, close the cap and store
the device according to the storage requirements until next use.
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
17 / 33
3.5. UNDERSTANDING THE TEST QUALITY
After each test session, quality grading will be displayed on the app to provide information
about how well the breathing maneuver was performed. Note that the acceptability of the
test is purely decided by the doctor/operator etc. This grade refers to the consistency of the
patient's maneuvers, not the health of the patient's lungs.
Grading of the FVC and FEV1parameters in children and adults, according to the American
Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines;
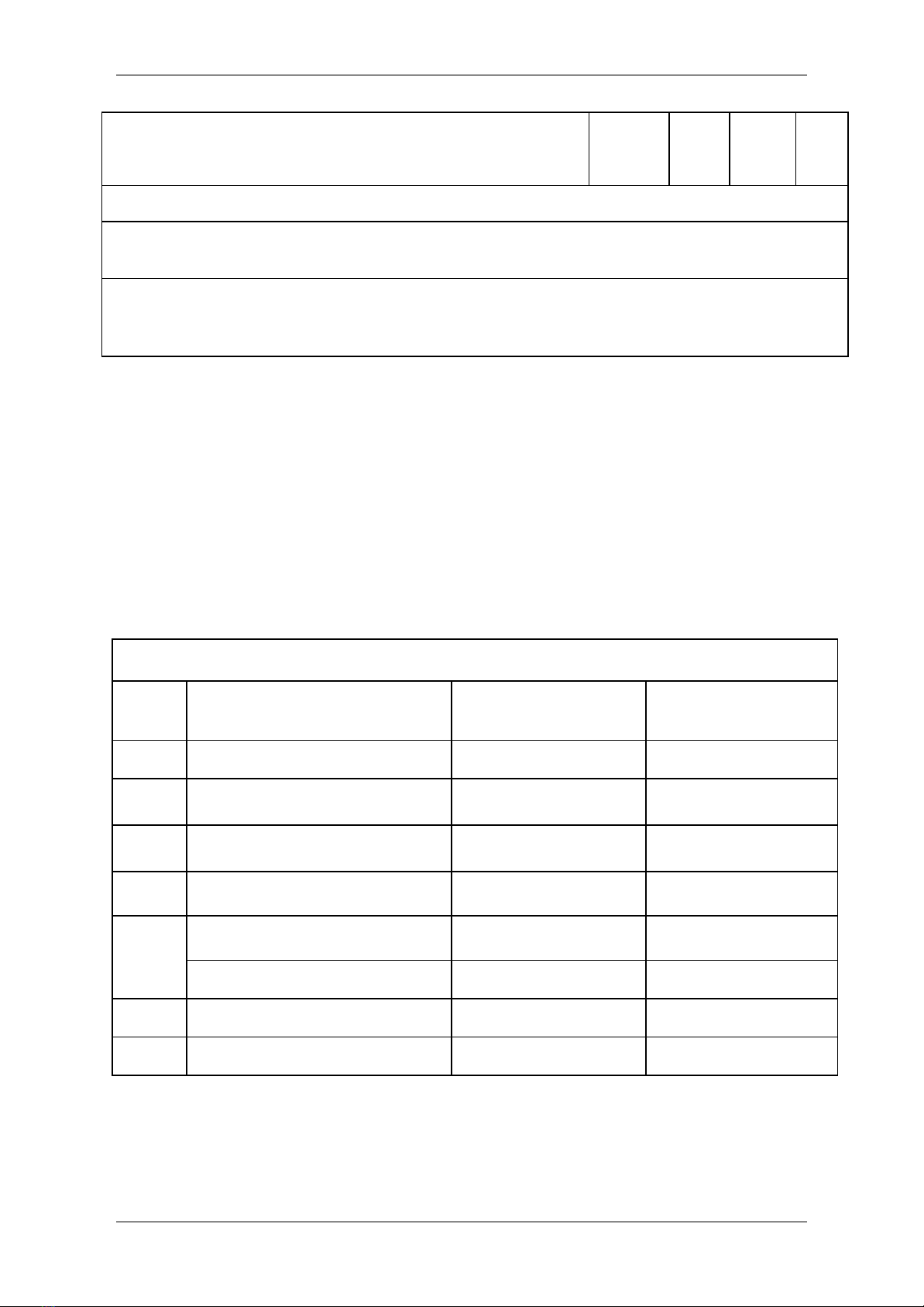
Summary of Acceptability, Usability, and Repeatability Criteria for FEV1
and FVC
Acceptability and Usability Criterion
Required for
Acceptability
Required for
Usability
FEV1
FVC
FEV1
FVC
Must have BEV ≤5% of FVC or 0.100 L, whichever is greater
Yes
Yes
Yes
Yes
Must have no evidence of a faulty zero-flow setting
Yes
Yes
Yes
Yes
Must have no cough in the first second of expiration
Yes
No
Yes
No
Must have no glottic closure in the first second of expiration*
Yes
Yes
Yes
Yes
Must have no glottic closure after 1 s of expiration
No
Yes
No
No
Must achieve one of these three EOFE indicators:
1. Expiratory plateau (≤0.025 L in the last 1 s of
expiration)
2. Expiratory time ≥15 s
3. FVC is within the repeatability tolerance of or is greater
than the largest prior observed FVC *
No
Yes
No
No
Must have no evidence of obstructed mouthpiece or spirometer
Yes
Yes
No
No
Must have no evidence of a leak
Yes
Yes
No
No
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
18 / 33
If the maximal inspiration after EOFE is greater than FVC, then
(FIVC — FVC) must be ≤0.100 L or 5% of FVC, whichever
is greater **
Yes
Yes
No
No
Repeatability criteria (applied to acceptable FVC and FEV1
values)
Age > 6 yr:
The difference between the two largest FVC values must be ≤0.150 L, and the
difference between the two largest FEV1
values must be ≤0.150 L
Age ≤ 6 yr:
The difference between the two largest FVC values must be ≤0.100 L or 10% of the
highest value, whichever is greater, and the difference between the two largest
FEV1
values must be ≤0.100 L or 10% of the highest value, whichever is greater
EOFE = end of forced expiration
* Occurs when the patient cannot expire long enough to achieve a plateau (e.g., children
with high elastic recoil or patients with restrictive lung disease) or when the patient inspires
or comes off the mouthpiece before a plateau. For within-maneuver acceptability, the FVC
must be greater than or within the repeatability tolerance of the largest FVC observed before
this maneuver within the current prebronchodilator or the current post-bronchodilator testing
set.
** Although the performance of a maximal forced inspiration is strongly recommended, its
absence does not preclude a maneuver from being judged acceptable, unless extrathoracic
obstruction is specifically being investigated.
Grading System for FEV1
and FVC (Graded Separately)
Grade
Number of Measurements
Repeatability:
Age >6 yr
Repeatability:
Age ≤6 yr *
A
≥ 3 acceptable
Within 0.150 L
Within 0.100 L *
B
2 acceptable
Within 0.150 L
Within 0.100 L *
C
≥ 2 acceptable
Within 0.200 L
Within 0.150 L *
D
≥ 2 acceptable
Within 0.250 L
Within 0.200 L *
E
≥ 2 acceptable
> 0.250 L
> 0.200 L *
OR 1 acceptable
NA
NA
U
0 acceptable AND ≥ 1 usable
NA
NA
F
0 acceptable and 0 usable
NA
NA
* Or 10% of the highest value, whichever is greater; applies for age 6 years or younger only.
NA: Not Applicable
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
spirohome
®
│Clinic
User Manual
19 / 33
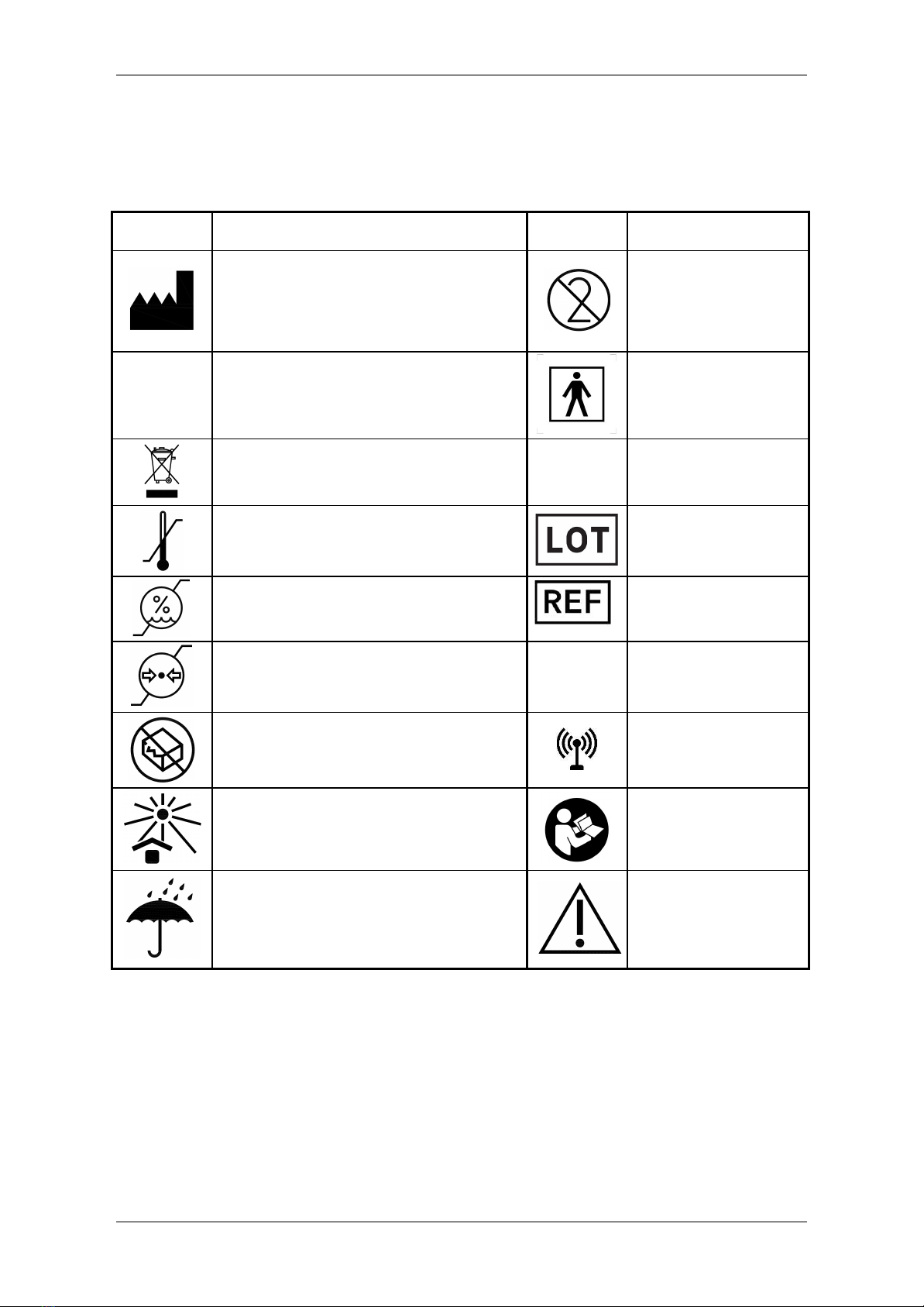
3.6. SIGNS AND SYMBOLS
Please note the following label, signs and symbols provided for the safe use and storage of
the Spirohome® Clinic.
Markings
Descriptions
Markings
Descriptions
“Manufacturer”
This symbol accompanied by the name
and the address of the manufacturer
adjacent to the symbol
Single-use only
Sign of Conformity
Type BF of Medical
Electrical Equipment
Disposal in Compliance with WEEE
SN
Serial Number
Temperature Limit
Lot Number
Humidity Limit
Ref Number
Atmospheric pressure limitation
IP
IP Number
Do not use if the package is damaged
The device includes
RF transmitters
Keep away from sunlight
The instruction
manual/booklet must
be read.
Keep dry
Caution
First Pub. Date: 24.09.2018
R.9-1 / 10.03.2020
This manual suits for next models
2
Table of contents