3
Introduction
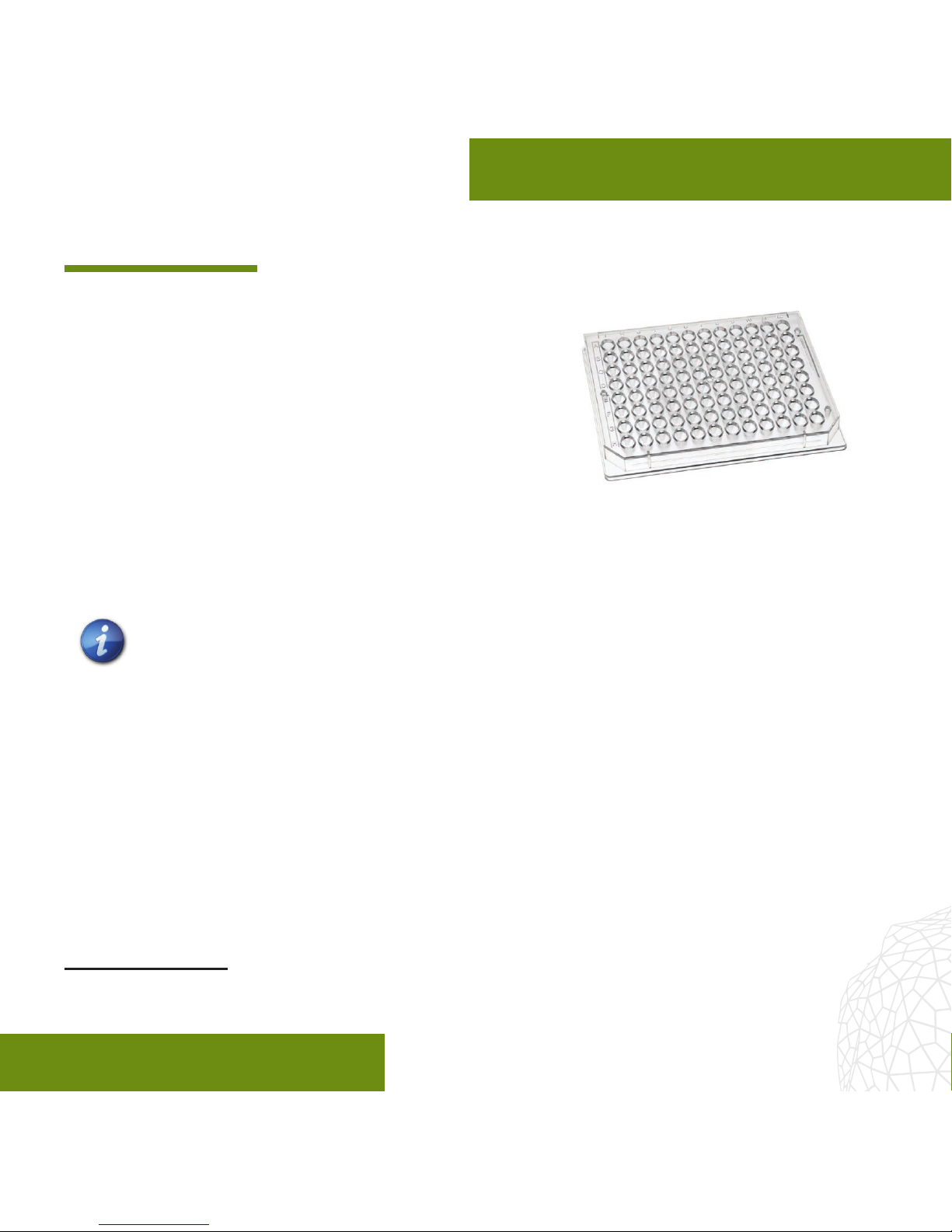
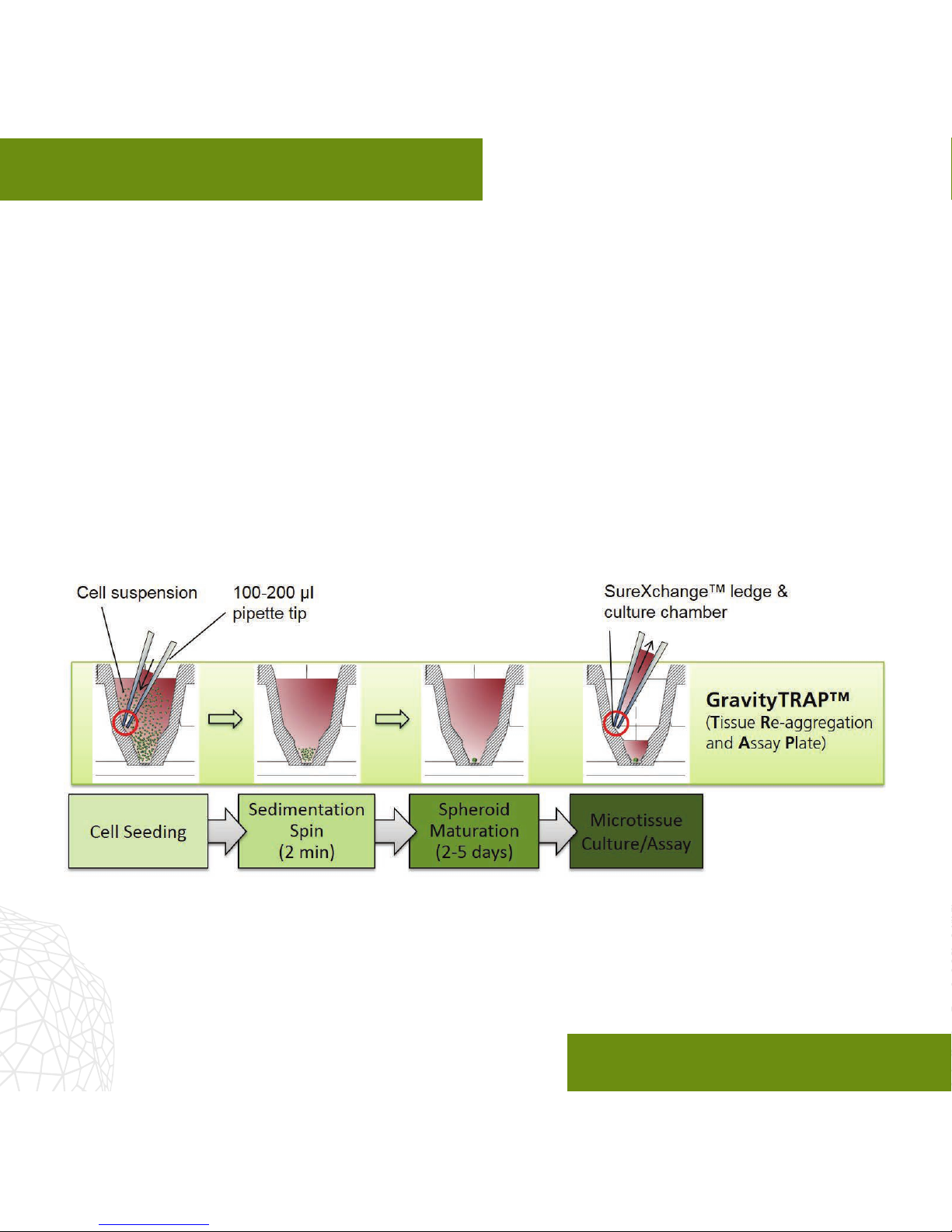
The GravityTRAP™ Ultra-Low Attachment
(ULA) plate1represents a simple, exible,
and automation-compatible platform for the
generation, long-term cultivation, observation
and testing of scaold-free 3D microtissue
spheroids in 96-well format. Each plate
consists of a special non-adhesively coated
96-well, sterile-packaged GravityTRAP™ ULA Plate and lid.
InSphero recommends GravityTRAP™ ULA plates for the generation of
spheroids using immortalized or modied cell lines that are known to
readily form microtissues, or as a starting point for investigating whether or
not a cell line can form self-aggregating, scaold-free spheroids. InSphero
recommends our patented GravityPLUS™ Hanging Drop System (ISP-06-
001, ISP-06-010) if generating spheroids in more complex 3D cell culture
scenarios, such as when using primary cells, cell lines that are sensitive to
self-assembly, or when generating co-culture microtissues (e.g., tumor/
stroma). In such cases, the GravityPLUS™ Hanging Drop System provides
the greatest opportunity for success.
1 The GravityTRAP™ ULA Plate and GravityPLUS™ Plate and related technology are protected by several granted and pending patents
world-wide.