InterX 1000 User manual
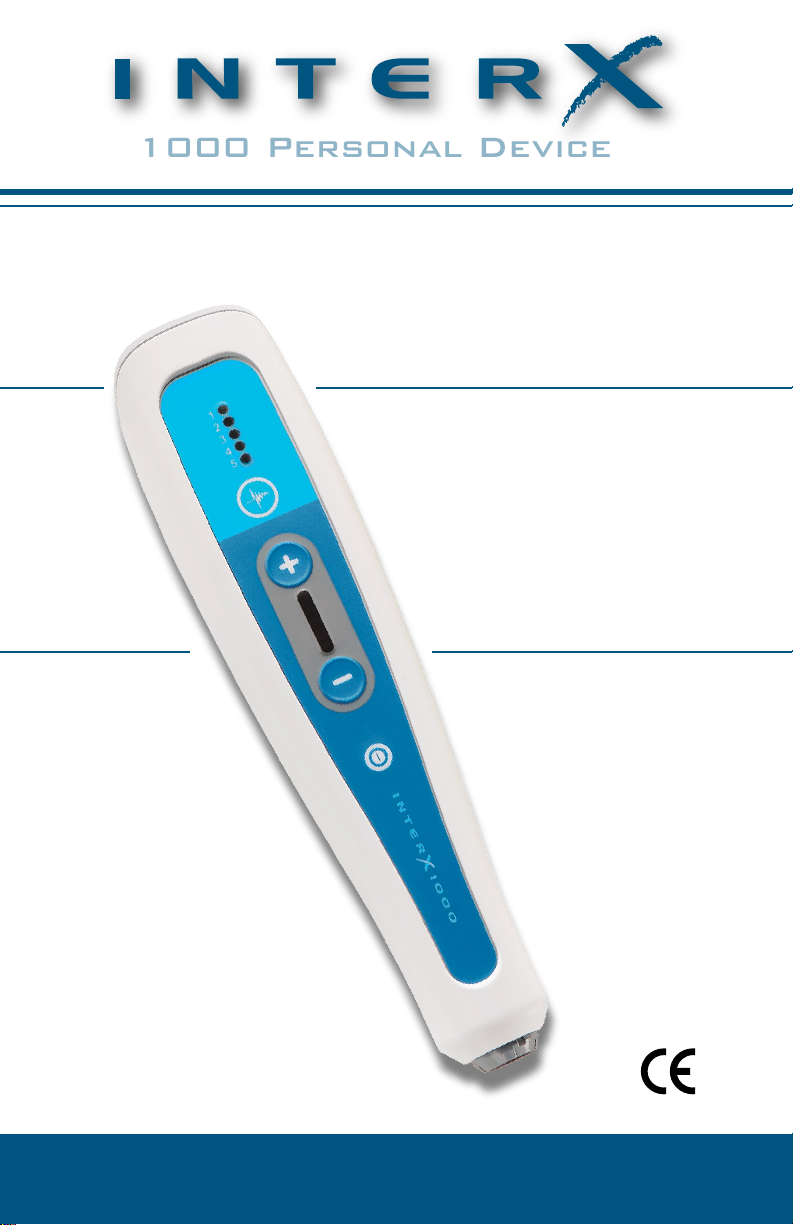
1000 Personal Device
OPERATIONS
MANUAL &
TREATMENT
GUIDE
®
Patent Pending
2797
US Patent No. 9,630,003 B2 and Patents Pending


Introduction
The InterX 1000 is designed to provide personal treatment of painful
conditions, like arthritis and also injuries and general aches and pains. The
InterX 1000 can be prescribed independently, or used in conjunction with
other therapies and as a support to treatment with the InterX professional
models.
The InterX 1000 provides an interactive response to the body’s changes
during recovery from injury or surgery. When applied to the skin, electrical
impulses adjust dynamically as the device encounters changes in the skin.
The InterX 1000 responds to the changes in skin tissue as it makes contact
through the electrodes and continues to adjust as the body begins to heal.
This interactive capability not only provides results, but also resists the
body’s natural tendency to develop a tolerance to static therapies.
Conveniently designed and completely portable, the InterX 1000 is a pain
management device suitable for individuals everywhere.
Please read this manual completely prior to using the InterX 1000.
Table of Contents
Operations Manual
Indications for Use ................................................................................3
Warnings and Cautions .........................................................................4
Definitions and Symbols........................................................................7
Overview of Controls and Functions......................................................8
Turning ON/OFF....................................................................................9
Preset Stimulation Patterns.....................................................................10
Set Stimulation Intensity ........................................................................11
Accessory Electrodes .............................................................................12
General Device Care
Battery Operation and Replacement .........................................14
Storage and Cleaning................................................................15
Service and Warranty ............................................................................16
Treatment Guide
Treatment Guidelines ............................................................................17
The Injury and Pain Curve .....................................................................18
Treatment Planning
Treating with the InterX 1000 ................................................................19
How to treat a Point of Pain ...................................................................20
How to treat an Area of Pain..................................................................22
Expanded Treatments
Increase Function and Activity ..................................................24
Ongoing Pain (General).............................................................26
Treating with the Flexible Array Electrode
Preset Stimulation Patterns ........................................................28
Treating an Area of Pain ............................................................29
Treating Ongoing Pain...............................................................31
Treating Ongoing Pain (Spinal) ..................................................33
Glossary / Definitions ............................................................................35
Troubleshooting Problems .....................................................................36
Product Specifications ...........................................................................37

InterX 1000
Operations Manual
This manual provides information regarding the controls and functions of
the InterX 1000. The InterX 1000 must be used strictly in accordance with
these instructions.
Indications for use
The InterX 1000 is indicated for:
• symptomatic relief and management of chronic intractable pain
• adjunctive treatment in the management of post-surgical and
post-traumatic pain.
The InterX 1000 carries the European CE mark for pain relief.
InterX Technologies is an ISO 13485 Registered company.
Contact InterX Technologies for country specific information or additional
regulatory approvals.
Definition –Warning: A WARNING message contains special safety
emphasis and must be observed at all times. Failure to observe a WARNING
message could result in serious personal injury.
Definition –Caution: Failure to observe a CAUTION associated with
use could result in minor injury or product damage. Such problems
include device malfunction, device failure, damage to the device or damage
to other property.
3
Any serious incident that occurs in relation to the device should be reported
immediately to the manufacturer and the competent authority of the Member
State in which the user and/or patient is established.

Contra-indications
• Electrode placement over malignant tumors
• Transcerebral and/or carotid sinus electrode placement
• Use over mucous membranes
• Undiagnosed pain (until etiology is established)
• Patients who are prone to seizures (e.g. patients with epilepsy)
• Use over pharyngeal or laryngeal muscles. The electrical impulses
generated may cause muscle spasm resulting in difficulty in breathing
• Patients that have a demand-type cardiac pacemaker
Warnings
Federal (U.S.A.) law restricts this device to sale by, or on the order of a
practitioner licensed by the law of the State in which he/she practices to use,
or order the use of the device.
Federal (U.S.A.) law requires the InterX 1000 be used only by a trained
healthcare practitioner or under the continued supervision of a licensed
healthcare practitioner. The InterX 1000 must be used only by the person for
whom it is prescribed. Electrode placement and stimulation settings should
be based on the guidance of the prescribing practitioner.
Safe use of the InterX 1000 is the primary responsibility of the user. The user
is responsible for the monitoring of the product. Contact clinical/technical
support if the InterX 1000 appears to be operating incorrectly.
The user must keep this device out of reach of children.
The InterX 1000 is not effective for pain of central origin including
headaches.
The InterX 1000 is a symptomatic treatment and as such could suppress the
sensation of pain which would otherwise serve as a protective mechanism.
The safety of the use of the InterX 1000 has not been established during
pregnancy or childbirth.
Do not operate the InterX 1000 before verifying that other medical devices
will not be adversely affected by the electrical impulses generated (e.g.,
electrical implants).
4

Warnings (cont.)
Stimulus delivered by this device may cause electrocution. Electrical current
of this magnitude must not flow through the thorax or carotid sinus nerves
because it may cause cardiac arrhythmia or interfere with cardiac function.
Use caution in applying the InterX 1000 over areas which are swollen,
infected, or inflamed as this may result in a worsening of symptoms. In
particular, caution should be taken when electrodes are placed over areas
associated with phlebitis, thrombophlebitis and varicose veins as these
conditions present an increased risk of forming blood clots which could
become dislodged during stimulation.
Use caution in applying the InterX 1000 to patients suspected of having
heart disease.
If the display becomes blank or inoperative, discontinue use.
Do not make contact with the InterX 1000 electrodes on wet skin. Natural
bodily fluids, including perspiration, are acceptable.
Extreme heat or cold may effect the operation of the InterX 1000.
Electronic monitoring equipment (such as ECG monitors and ECG alarms)
may not operate properly when stimulation is in use.
Do not use on patients that are undergoing dialysis or are being treated in an
MRI, X-ray, or with other diagnostic equipment that may be impacted by the
electrical impulses. Remove all jewelry before treatment.
The InterX 1000 is not to be used in the presence of anesthetic or other
flammable gases.
The InterX 1000 has no curative value.
Avoid placing the device on the skin when turning on or returning from
pause to avoid electrical signal.
Treatments with the InterX 1000 should not exceed 1 hour in any specific area
of the body and there should be a minimum of 2 hours between treatment
sessions, to avoid isolated cases of skin irritation.
Skin irritation, bruising, electrode burns, dizziness, nausea, and headaches
are potential adverse reactions.
5

Cautions
The InterX 1000 should be used only with manufacturer approved electrodes
and accessories. Built-in device electrodes and external electrodes should
not be used in combination transcerebrally.
Avoid spilling fluids on the device. If the InterX 1000 is immersed in any
liquid it must be replaced with a new device.
Do not sterilize the InterX 1000.
Do not expose any part of the InterX 1000 to chemical solvents or harsh
cleaning fluids. Follow cleaning instructions in this manual.
Effectiveness of the InterX 1000 is highly dependent upon patient selection
by a person qualified in the management of pain.
The InterX 1000 should not be used while driving, operating machinery, or
during any activity which may put the user at undue risk of injury.
Do not open the InterX 1000 case. Opening or removing the housing
may expose you to dangerous voltage or other hazards and can damage
operating circuits. Opening the case will void the manufacturer’s warranty. If
the device should need repair or service contact InterX Technologies, your
InterX 1000 distributor or an authorized InterX service representative.
Turn device OFF before replacing batteries to avoid unexpected electrical
signal. Only the battery cover may be removed when changing batteries. Do
not attempt to connect the InterX 1000 to any other power source.
6

Definitions and Symbols
This CE symbol certifies that the product complies with the
essential requirements of the Medical Device Directive.
The “NRTL/C” indicator adjacent to the CSA (Canadian
Standards Association) mark signifies the product has
met the applicable ANSI/UL and CSA standards for use
in the U.S. and Canada. NRTL (Nationally Recognized
Testing Laboratory) is a designation granted by the U.S.
Occupational Safety and Health Administration (OSHA)
to laboratories which have been recognized to perform
certification to U.S. Standards.
This stimulator is internally powered only. The symbol
indicates the device was manufactured according to the
degree of protection against electrical shock for this type BF
protection class equipment.
DO NOT use the InterX 1000 without reading this manual.
The Serial Number (SN) and the
manufacturing information are located
on the battery label inside the battery
compartment.
C US
®
7
2797
InterX Technologies
870 N Dorothy Dr. Ste 708
Richardson, TX 75081 USA
2X 1.5V DC
(01) 00851894007022
(21) 1149XXXX 1000
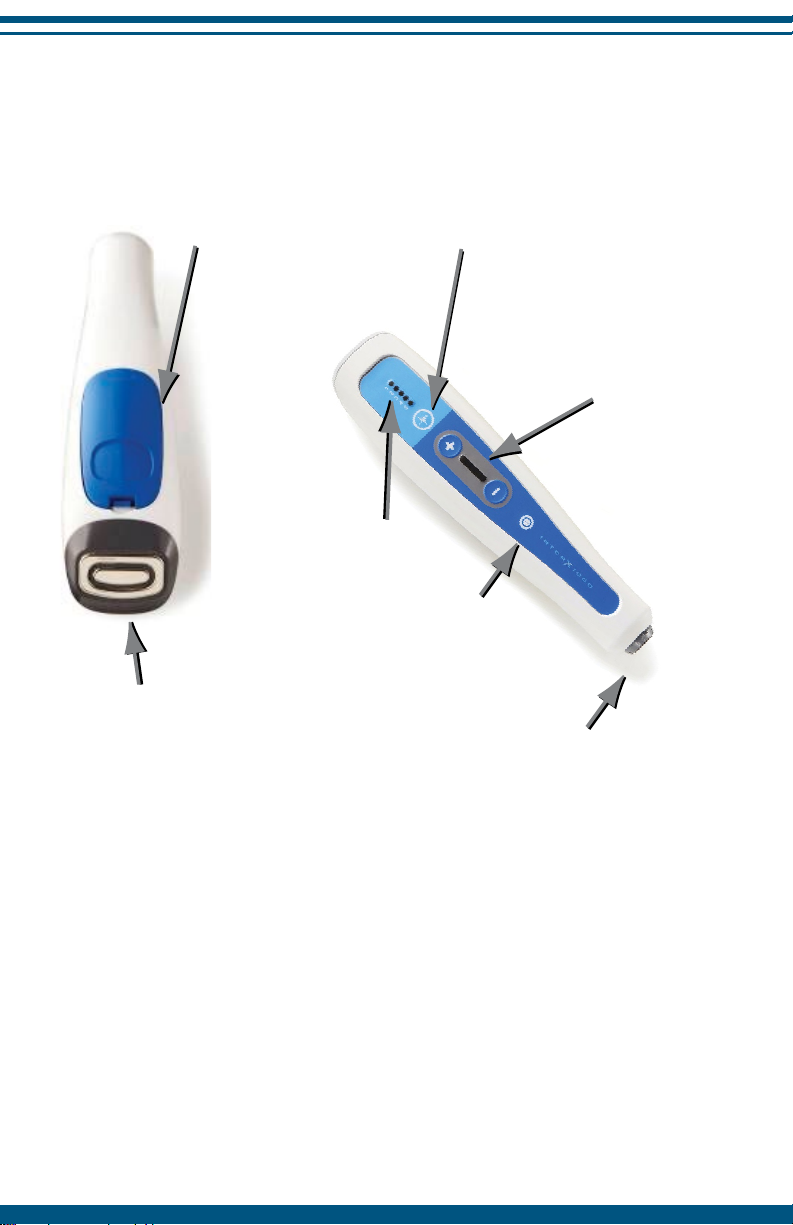
Overview of Controls and Functions
8
Preset Stimulation
Select Button
Preset LED
Indicator
Accessory
Electrode input
On/Off
Button
Intensity Level
(+ Increase; - Decrease)
Battery
case
Main Electrode

Turning the InterX Personal Sport ON/OFF
To begin InterX therapy, turn the device
on by pushing the ON/OFF button, with the device off the skin.
Upon start-up the instrument goes through a short self-test and
then a short audible beep will be heard. The amber LED for
Preset 1 will glow to show the device is turned on. To turn the
InterX 1000 off, press and hold the ON/OFF button
until an audible beep is heard. Wait 5 seconds before placing
the device on the skin.
The InterX 1000 may not perform correctly if:
1. The battery is dead.
2. An incompatible electrode is plugged in.
3. There is a device failure.
ON/OFF
Button
+
_
-
o
9
1
2
3
4
5
Selecting a Preset Stimulation Pattern
There are five preset stimulation patterns on the InterX 1000. Press the
PRESET button until you have reached the desired stimulation setting. An
amber LED will light to show the preset stimulation pattern that is active.
Your physician or therapist may provide you with the most appropriate
preset stimulation pattern for your condition.
PRESET button
Preset 1 : 60 PPS (pulses per second)
This preset is appropriate for ongoing pain and persistent
conditions.
Preset 2 : 15 – 60 PPS
Low to moderate stimulation setting. This variable impulse is
recommended for conditions that are moving towards recovery and
ongoing pain conditions.
Preset 3 : 30 – 120 PPS
Moderate to high stimulation setting. This variable impulse is
recommended for new pain resulting from an injury or recent
surgery.
Preset 4 : 240 PPS
High stimulation setting. This preset is recommended for higher
pain levels and when a new painful condition has recently
occurred.
Preset 5 : 480 PPS in bursts
The highest stimulation setting. This preset is recommended for the
immediate treatment of an injury, and is a strong stimulation.
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
10
Preset SELECT button
How to Set Stimulation Intensity
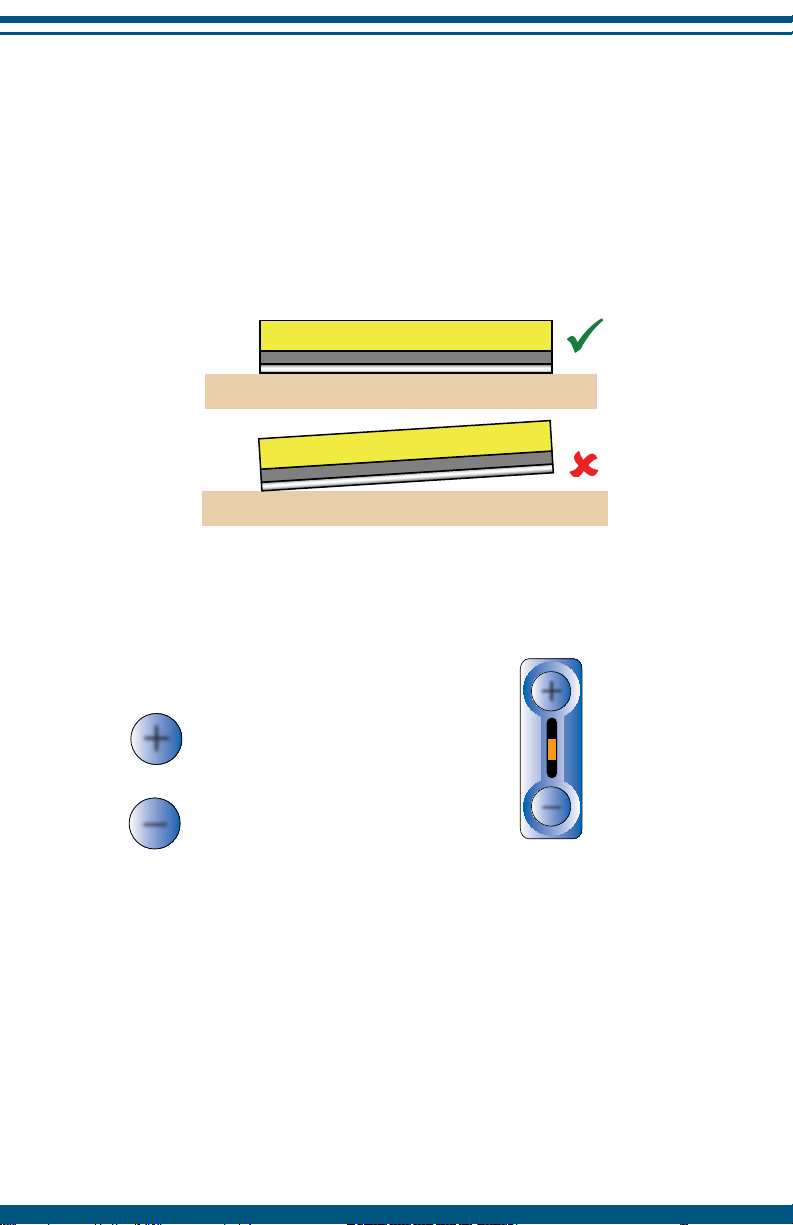
Place Electrode on the Skin
Ensure the InterX 1000 electrodes are in full contact with the skin on or
near the area to be treated before increasing intensity. The sensation from
the InterX 1000 will vary from place to place and day to day so it is VERY
important to only increase intensity when the device is on the skin.
NOTE: It is recommended that the skin remain in a “natural” condition.
Creams and lotions should not be used and excess perspiration should be
wiped away.
Set Stimulation Intensity
Press to increase the intensity to
a comfortable tingling sensation.
Press to decrease the intensity if the
stimulation is uncomfortable.
NOTE: While changing the intensity,
the preset LED will flash.
The intensity level should be started at a minimum level and gradually
increased until you feel a comfortable tingling sensation. It is not necessary
to use a high level of stimulation.
The amber LED displays the level of intensity as it is changed. This will only
be displayed while you are changing the level of intensity and for three
seconds afterwards.
ON/OFF
Button
+
_
-
o
ON/OFF
Button
+
_
-
o
+
_
Intensity
Display
11
Good Skin Contact
Poor Skin Contact

Description of Accessory Electrodes
There is a range of accessory electrodes available for use with the InterX
1000. The accessory electrodes plug into the accessory port of the InterX
1000 located at the end of the device (opposite end to the main built-in
electrode). Use care when plugging in the lead wire noting the alignment
guide for connection. To remove, hold the insulated connector and gently
pull apart.
NOTE: Jerking the lead wire instead of holding its insulated connector may
cause damage.
Do not attempt to plug other devices or accessories into the accessory port
of the InterX 1000. Only manufacturer approved electrodes may be used
with the InterX 1000. The electrode package contains instructions for care
and replacement of accessory electrodes.
When an accessory electrode is plugged or unplugged, the device will
default to preset 1 and minimum stimulation intensity.
The main electrode will be inactive when an accessory is plugged in. Use
the accessory electrode in the same way as the main built- in electrode is
used.
NOTE: Some accessory electrodes will provide different stimulation
patterns based on intended use.
All instructions in this manual apply to user placement of both built-in and
external electrodes.
Insulated connector Alignment guide
Accessory electrode
connection port
12

19
Accessory Electrodes
for use with the InterX 1000
The Dome Electrode –
designed specifically to
cover a larger area of skin tissue.
The Soft-Tissue Electrode –
designed for use on muscles,
myofascial release, or massage tool.
The Comb Electrode –electrode is
designed for use on areas of skin
where hair is thicker and on the scalp.
The Point-stim and Activity Reading
(AR) features are not
active when this electrode is in use.
Stimulation is continuous
regardless of skin contact.
The Flexible Array™–
designed to provide 10 minute
treatment options and can be used
unattended once treatment
parameters have been set. It comes in
two sizes: 3x3 dual pads
and 4x4 single pad.
The Cosmetic Electrode –
primarily used as a facial treatment.
The Classic Electrode –
for use on smaller areas, such as the
face, neck, hands, and feet.
The Personal Flexible Array –
designed to be thin and more flexible
around bony points, providing good
contact. Numeric readout of
impedance is available on the InterX
5002.
13

General Device Care
Battery Replacement
The InterX 1000 operates by battery power only. Use new, quality AA
alkaline batteries for longer life and optimum performance of the device.
Rechargeable batteries may be used. Ensure that these are fully charged
before use.The InterX 1000 is rated for continuous operation.
Battery life is highly dependent on how often the device is used and the
specific settings that are used for treatments. However, under normal use
(approximately 1-2 hours per day at varying degrees of power ) battery life
of the device is approximately 4 weeks.
Low Battery Condition
When the battery is low, a tone will sound and the LED will flash. If this
happens, the batteries should be replaced to continue use. The device will
continue providing stimulation, but will periodically make a descending
tone to warn of a low battery condition until the batteries are changed.
If the battery becomes completely depleted the device will emit the low
battery tone and then turn off automatically and will not restart. You MUST
replace the batteries to continue use.
14
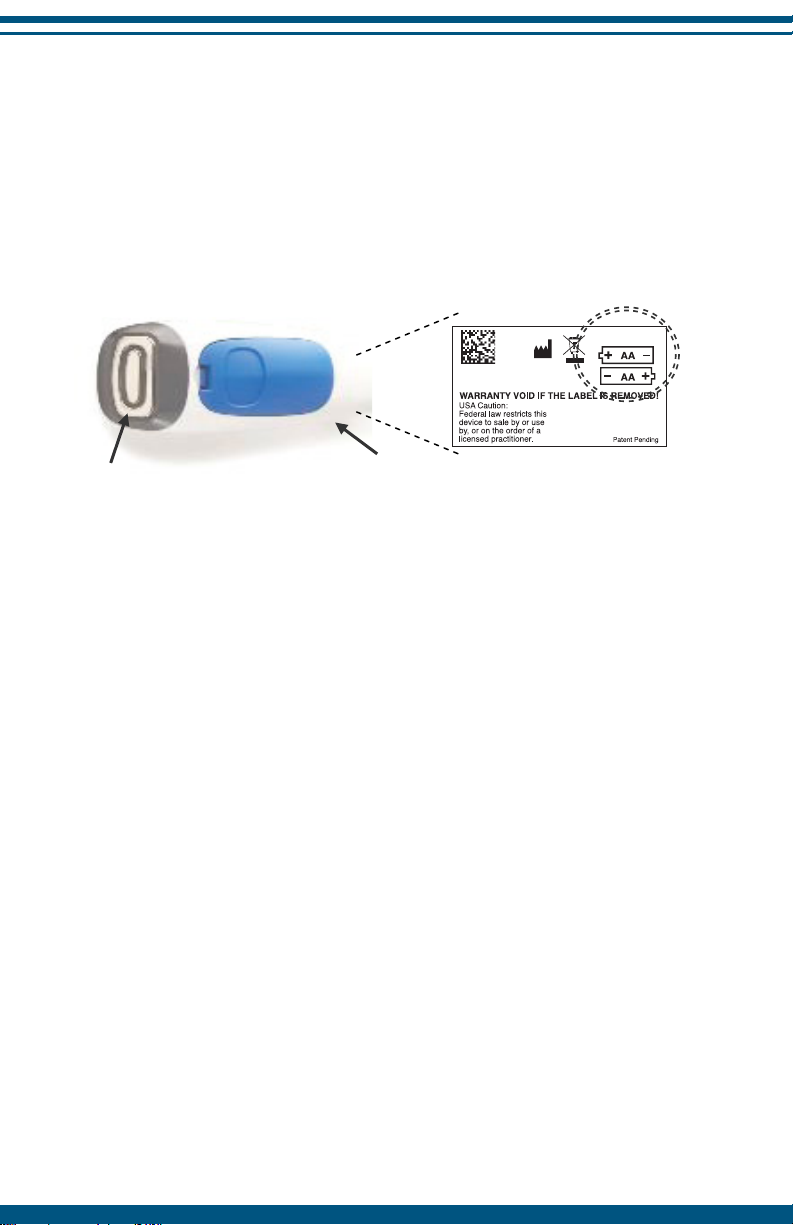
Removing and Replacing Batteries
To remove the batteries, open the battery case and take out the old
batteries. Properly dispose of the old batteries and replace with fresh, new
AA alkaline batteries as indicated below. Securely replace the battery cover
back on the device by pushing until it snaps in place. The device will not
function if the batteries are placed in the compartment incorrectly.
15
Battery
compartment
Storage and Cleaning
Remove the battery when storing the InterX 1000 for more than one month.
Always transport the InterX1000 with care. When not in use, store the InterX
in dry conditions.
Clean the InterX 1000 and accessory electrodes with the main power OFF.
The InterX 1000 is a non-critical contact device indicated only for contact
between the electrodes and intact skin. Between treatments, thoroughly
clean the main electrode, accessory electrodes, and surrounding device area with
70% isopropryl alcohol wipes. Use of other cleaning solutions may damage
the housing. Never spray cleaners directly on the device.
CAUTION: Do not use cleaning products that contain ethyl alcohol and/or
ammonium chloride. These chemicals may cause cracking of the plastic. The
only approved cleaning agent is isopropyl alcohol that is less than or equal to
90% by volume.
Using unapproved cleaning agents will void the manufacturer’s warranty.
InterX Technologies
870 N Dorothy Dr. Ste 708
Richardson, TX 75081 USA
2X 1.5V DC
(01) 00851894007022
(21) 1149XXXX 1000
Main
Electrode

Service and One-Year Limited Warranty
The InterX 1000 is not user-serviceable. Never attempt to open the housing as
this device contains high voltages during operation. All warnings, cautions, and
instructions contained in this manual must be followed to ensure full warranty
coverage.
To obtain service, contact InterX Customer Service at (1) 972-665-1810, for a
Returned Goods Authorization (RGA) number. Send the entire unit, with all
accessories (if applicable), packed in the original carrying case, freight and
insurance prepaid to the address provided to you by InterX. Include in the package
a copy of your original invoice and a note describing the problem. Be sure to
include your return address, phone number, fax number and/or an email address, if
available.
InterX Technologies will not be responsible for damage due to improper packaging
or shipment.
InterX Technologies warrants to the original purchaser that each new InterX 1000
is free of defects in workmanship and materials under normal use for a period of one
year from original purchase date, except for the battery and carrying case.
During the warranty period, our sole obligation shall be, at InterX Technologies
option, to repair or replace the InterX 1000 without charge. If the InterX 1000 is
outside the warranty coverage period any requested repairs or replacement charges
will be invoiced to the customer.
If InterX Technologies determines there is a defect covered by this warranty, the
repaired or replaced product will be shipped back, freight and insurance prepaid. If
InterX determines, in its judgment, that the product does not contain defective
workmanship or materials, InterX Technologies will return the product and invoice
the customer for the repairs, return freight and insurance charges.
The warranty is voided immediately if the product has been subjected to abuse,
accidental damage, damage in transit, negligence, acts of nature, or damage
resulting from failure to follow operating instructions, or alteration/disassembly by
anyone other than InterX. Opening of the InterX 1000 case will void the warranty.
InterX shall not be liable for any direct, indirect, special, incidental, or consequential
damages, lost profits or medical expenses caused by any defect, failure, malfunction,
or otherwise of the product, regardless of the form in which any legal or equitable
action may be brought against InterX Technologies (such as contract, negligence, or
otherwise). In no event shall InterX Technologies liability under any cause of action
relating to the product exceed the purchase price of the product. Repair or replacement
of the device under this warranty will not extend the original warranty time period.
Batteries and carrying cases, are excluded from the warranty and are sold as is.
16

17
InterX 1000
Treatment Guide
Treatment Guidelines
Treatment should be focused on the point or
area where the pain is felt. Begin stimulation
at the site of the injury and/or pain.
The skin and tissue around the injury/pain site is
often impacted by the damage, therefore stimulation
to surrounding skin is also recommended.
Treatment can be expanded to other areas if the pain
does not resolve; see treatment instructions for
ongoing pain (pg. 26).
If necessary, wipe away any excess perspiration.
The InterX 1000 is unlike other electrical stimulation
products:
• Do not exceed the recommended treatment time
• The treatment Intensity level will vary from treatment to
treatment or within one treatment session. DO NOT allow
the stimulation to be uncomfortable or painful
• Certain conditions and injuries may require professional treatment
and appropriate advice and information should always be sought in
these circumstances
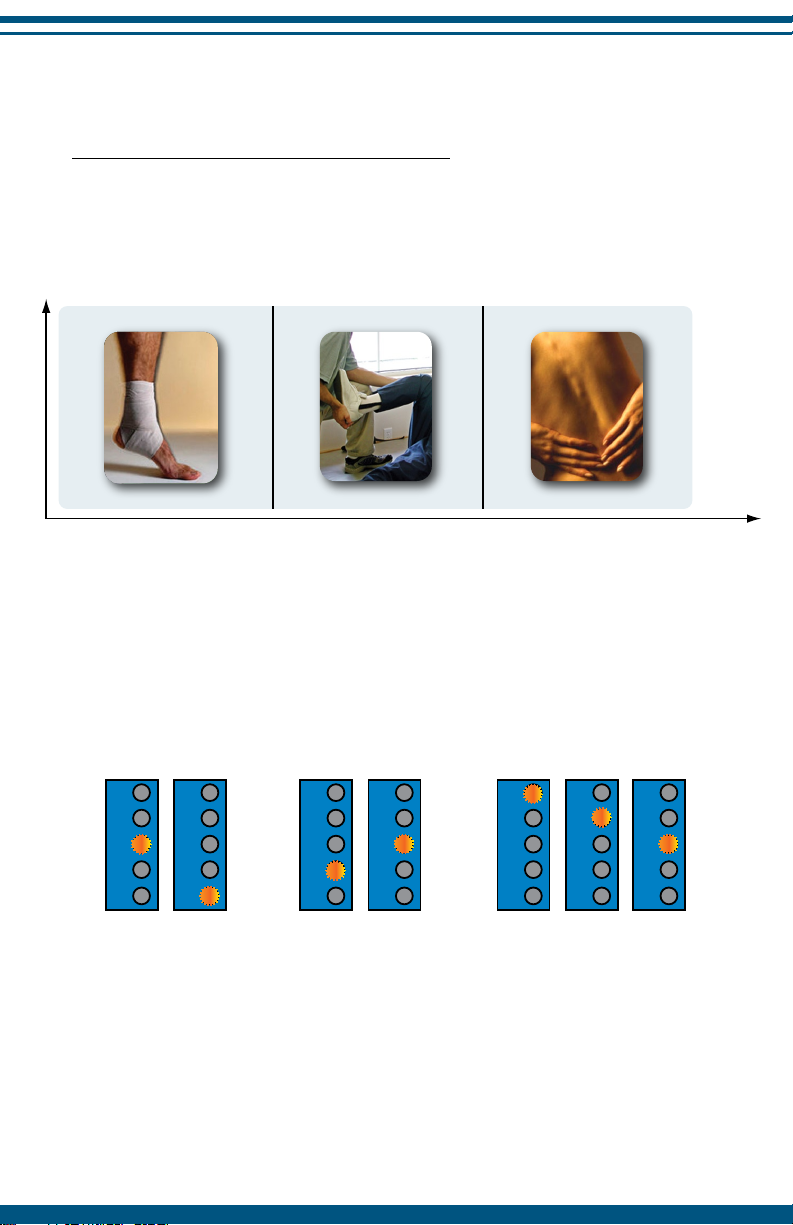
Stages of Condition and Pain
Treatment of pain in different stages
Different preset stimulation patterns are available to treat different
conditions and pain stages. The illustration below is a guide to the preset
selections and general time periods for treatment.
Recommended Presets
18
New
Pain resulting from
an injury (sprain,
strain, bruise) or
recent surgery.
Recovery/Rehab
Pain associated
with return to
normal activities
or increasing
function.
Ongoing
Pain or injury that
has persisted for 3
months or longer.
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
1
2
3
4
5
New
2 Weeks 2 Weeks - 3 Months > 3 Months Time
Recovery/Rehab Ongoing
Other manuals for 1000
1
Table of contents
Other InterX Medical Equipment manuals