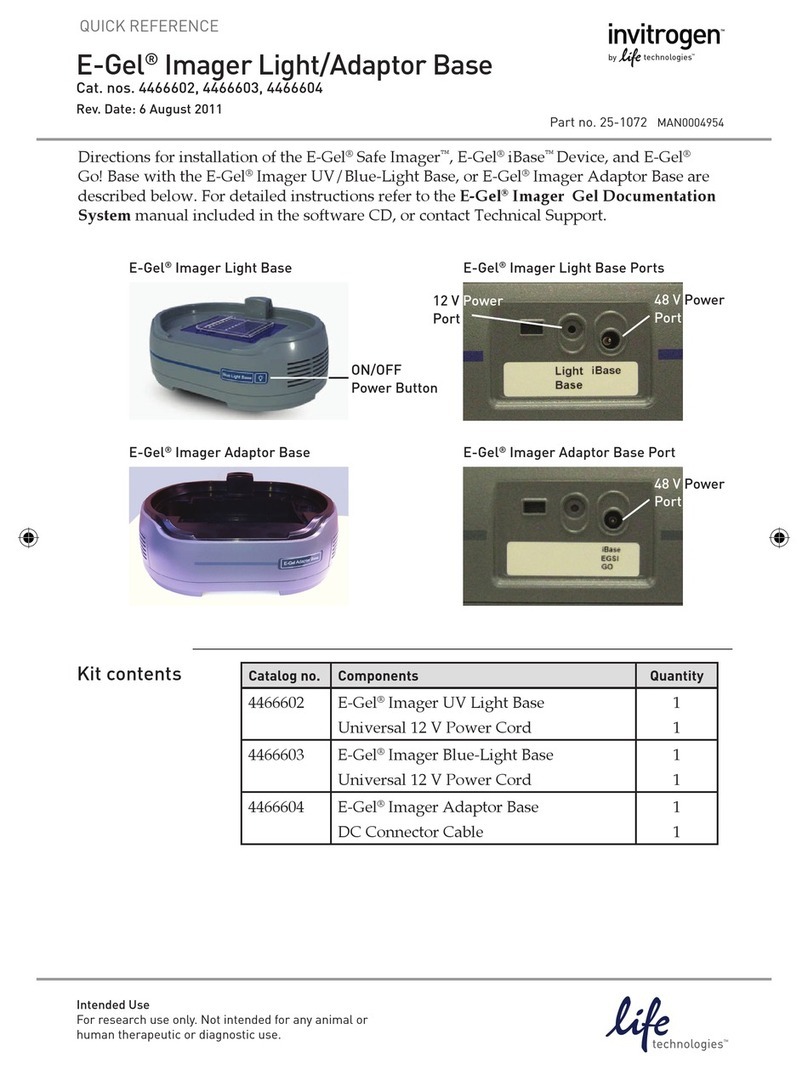
For support visit thermofisher.com/support or email techsupport@lifetech.com
thermofisher.com
12 December 2015
© 2015 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
DISCLAIMER: LIFE TECHNOLOGIES CORPORATION AND/OR ITS AFFILIATE(S) DISCLAIM ALL WARRANTIES WITH RESPECT TO THIS DOCUMENT, EXPRESSED OR IMPLIED, INCLUDING BUT NOT LIMITED TO
THOSE OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. TO THE EXTENT ALLOWED BY LAW, IN NO EVENT SHALL LIFE TECHNOLOGIES AND/OR ITS AFFILIATE(S) BE
LIABLE, WHETHER IN CONTRACT, TORT, WARRANTY, OR UNDER ANY STATUTE OR ON ANY OTHER BASIS FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CON-
NECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING BUT NOT LIMITED TO THE USE THEREOF.
Important licensing information: This product may be covered by one or more Limited Use Label Licenses. By use of this product, you accept the terms and conditions of all ap-
plicable Limited Use Label Licenses.
Corporate entity: Life Technologies | Carlsbad, CA 92008 USA | Toll Free in USA 1.800.955.6288
Limited Product Warranty:
Life Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies’ General Terms and Conditions of Sale found on Life Technologies’
website at www.lifetechnologies.com/termsandconditions. If you have any questions, please contact Life Technologies at www.lifetechnologies.com/support.
Mini Blot Module
Cat. no. B1000
Instructions for using the Mini Blot Module to transfer proteins onto a membrane are described below. For detailed instructions, refer to the
manual available from thermosher.com. See reverse side for instructions on using the Mini Gel Tank for electrophoresis.
Before Starting
• Select transfer membrane appropriate for your purpose, and prepare it for transfer (refer to Mini Blot Module manual for details).
• Prepare 250 mL of 1X transfer buffer for each transfer.
• Soak two pieces of lter paper briey in 1X transfer buffer.
• Soak two sponge pads thoroughly in 1X transfer buffer. Squeeze submerged pads to ensure that air bubbles are removed.
Note: It is important to use clean sponge pads to avoid protein contamination. (refer to the manual for details on care of sponge pads).
• Trim wells and foot from gel.
Transfer Conditions
Transfer protein (using 1 or 2 blot modules) for 60 min at a constant voltage of 10 V (nitrocellulose) or 20 V (PVDF). Do not exceed 30 V.
Anode core (+)
Cathode core (–)
Sponge pad
Sponge pad
Filter paper
Filter paper
Membrane
Gel
35
Order of assembly
4
3. Make sure any cassette clamps are
removed from the chambers.
Insert the blot module with the
cathode core (–) facing the front.
The blot module should be seated so
that the electrode makes contact with
the cathode.
4. Add 1X transfer buffer to the module
core if the sandwich is not completely
submerged.
Add deionized water or 1X transfer
buffer (~225 mL) to the chamber up to
the level of the cathode.
5. Make sure the power supply is off.
Place the lid on the tank and plug the
electrode cords into the power supply.
Turn the power supply on to begin
transfer.
Module Core
Chamber
Front
Cathode
Electrode
Cathode
Protein Transfer Protocol
1. Place the cathode core (–) on a flat surface, and assemble the
sandwich according to the diagram (right).
• Only one gel can be transferred at a time in a single module.
• Always handle the membrane with the Blotting Tweezers.
• Use the Blotting Roller to remove any bubbles between
layers of the sandwich.
2. Place the anode core (+) on top of the sandwich, and close the
module assembly.
Fill