N.B. The heater is not intended to hold the dish at precisely 37°C. Rather its
purpose is to slow cooling when the dish is removed from an incubator.
When required by the workflow, user-supplied reagents must be placed in 1.5ml
microcentrifuge tubes (supplied in the CloneG consumables kit) at six defined
locations on the bed (Figure 2). A minimum of 1ml of each required reagent should be
provided. Note that:
The isoCell will provide a visual prompt of the reagents required by any given
part of the workflow.
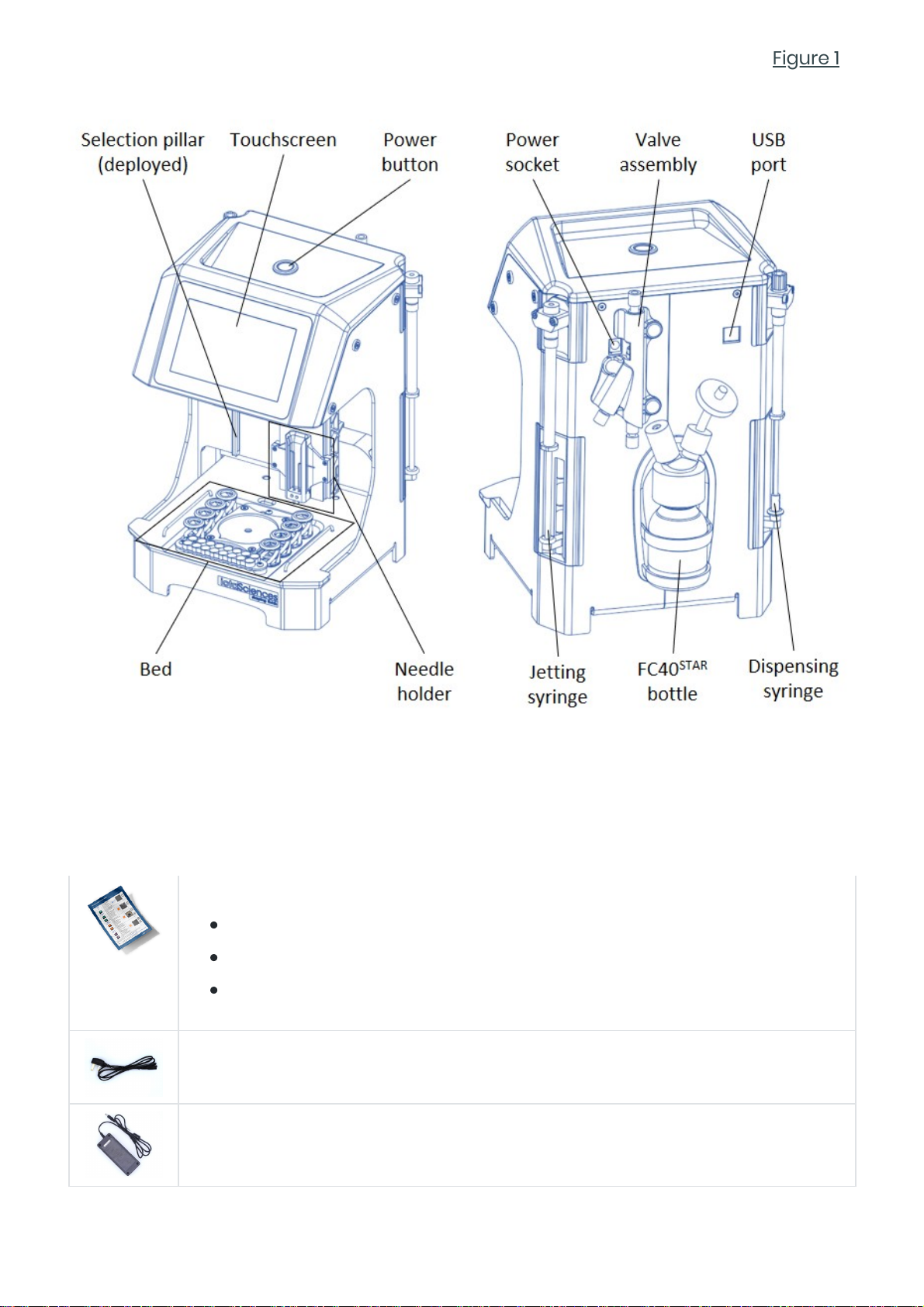
A seventh reagent, FC40STAR, is stored in the FC40STAR bottle at the rear of the
isoCell (Figure 1).
Before beginning any program, ensure all microcentrifuge tubes are open
with their lids tucked under the rails, as shown in Figure 2. This is essential to
prevent obstruction of the needle holder.
Two further (empty) microcentrifuge tubes serve as repositories for aqueous and
FC40STAR waste. The isoCell will provide a visual prompt when waste microcentrifuge
tubes are required. Users should ensure that waste tubes are empty.
For instructions on the safe disposal of FC40STAR, refer to
Disposal of FC40STAR
.
Cells are harvested by the isoCell from the 60mm dish into the two 8-tube PCR strips
(or flat bottom 8-well strips) located at the front of the bed (Figure 2). The isoCell will
provide a visual prompt when the PCR or flat bottom strips are required. The strips
should be prepared with 120µl cell culture medium in each tube/well.
Do not put caps on the PCR strips.
Running programs
The isoCell workflow of isolating, culturing and harvesting cells is executed by
running a series of programs on the isoCell. For instance, the
GRID
program will
create a GRID on the 60mm dish, the
plate
program will dispense cells into a GRID,
etc. All programs are executed from the isoCell’s interactive touchscreen. A stylus is
supplied for higher touch accuracy when required.
At the beginning of each program, a message on the touchscreen will prompt the
user to place the correct materials and reagents onto the
isoCell bed
. When
providing reagents in microcentrifuge tubes, the user should fill the tube to a