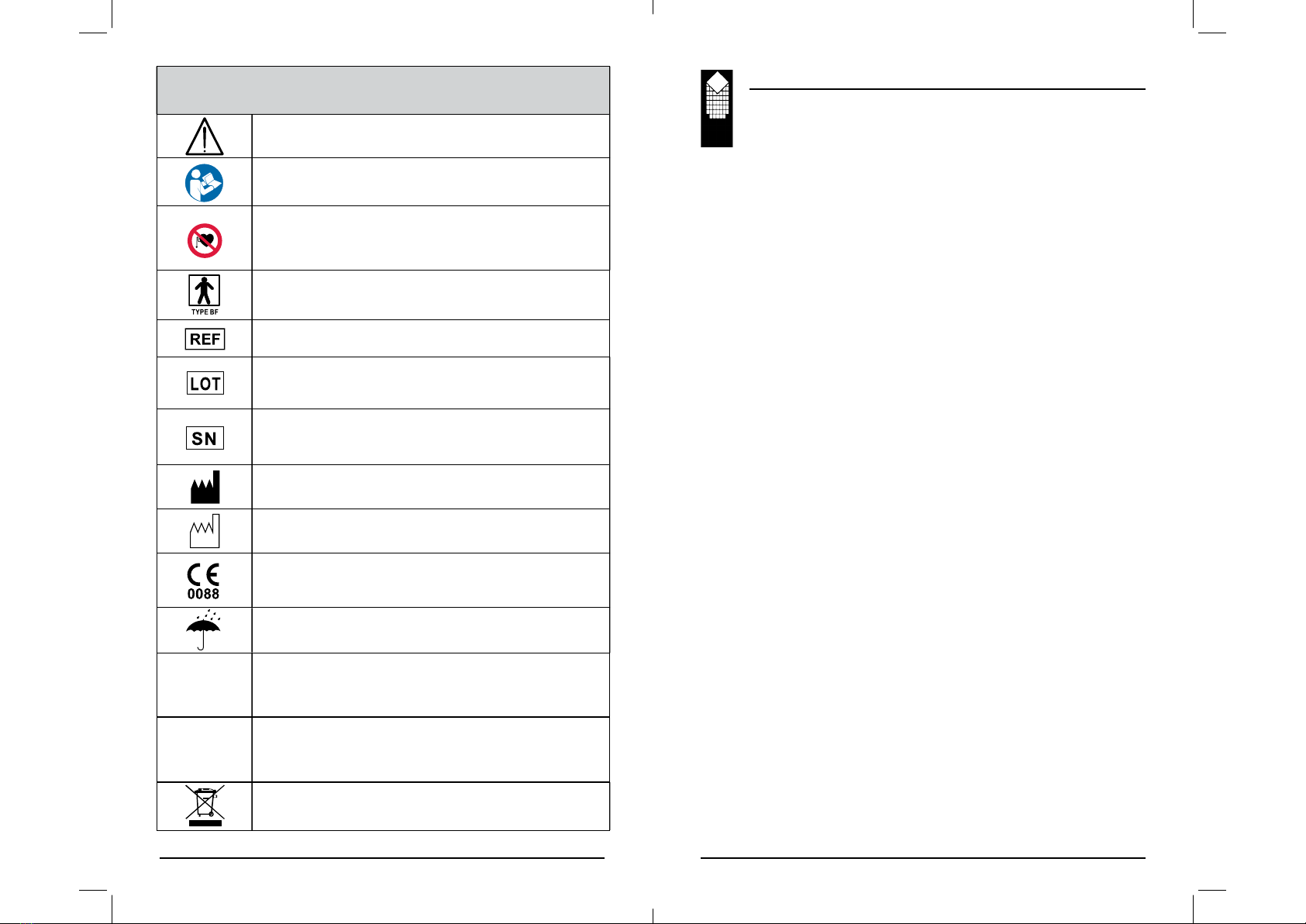
Kegel8® Mother Nurture Operation Manual
6
Please read this Kegel8 Mother Nurture User Guide before using this
stimulation device.
Kegel8 Mother Nurture should not be used:
• By patients fi tted with a demand style cardiac pacemaker unless
advised to by their Doctor.
• On a STIM programme during pregnancy (unless medically
advised).
• On a TENS programme prior to 37 weeks pregnancy.
• By patients with undiagnosed pain conditions.
• By patients with undiagnosed skin, vaginal or rectal conditions.
• With patients who have diminished mental capacity or physical
competence who cannot handle the device properly.
• On anaesthetised or desensitised skin.
• When driving a vehicle or operating potentially dangerous
equipment.
When using Kegel8 Mother Nurture:
• Use only as directed.
• Do not immerse the unit in water or any other liquid.
• Keep unit out of reach of children.
• Seek professional advice if you are unsure about using Kegel8
Mother Nurture. Contact your distributor or healthcare professional.
• Only use CE approved skin electrodes and vaginal probes.
• Change your skin electrodes to hypoallergenic electrodes should you
experience any irritation from the adhesive coating on the electrodes
supplied. A range of skin electrodes for sensitive skin can be found
on our website.
• Allow the skin to heal should you experience any skin irritation
before commencing use.
Do not place electrodes:
• Over carotid sinus nerves, larynx or trachea.
• Inside the mouth.
• Over the area of the heart unless so advised by your doctor.
• On your facial area unless under strict guidance from a qualifi ed
clinician.
• Do not apply stimulation across or through the head, directly on the
eyes, covering the mouth, on the front of the neck (especially the
carotid sinus) or via electrodes placed on the chest and upper back or
crossing over the heart.
Contra Indications & Precautions
51
Kegel8® Mother Nurture Operation Manual
Warranty
We provide a warranty to the original purchaser, that this product will
be free from defects in the material, components and workmanship, for
a period of 2 years from the date of purchase by the distributor.
If the distributor is satisfi ed that the product is defective, the user may
return the unit directly to Savantini Limited. All returns must be au-
thorised and the warranty does not extend to any misuse or abuse such
as drop ping or immersing the unit in water or other liquid substance
or tampering with the unit or normal wear and tear. Any evidence of
tampering will nullify this warranty.
Customer Service and Distribution:
Please contact your distributor for any customer service enquiries,
including the warranty returns. Your invoice of purchase and/or the
rear cover of this manual should state the name and the contact details
of your distributor.
For assistance, if needed, in setting up, using or maintaining the unit,
or report unexpected operation or events, please visit the distributor’s
website for further details: www.kegel8.co.uk
Distributed by: Savantini Ltd.
Savantini House, Foster Street
Stoneferry Road, Hull
HU8 8BT, UK
Fax: +44(0) 1482 873570
Email: sales@kegel8.co.uk
Web: www.kegel8.co.uk
Helpline:
+ 44(0) 1482 496 932
This product is manufactured by Verity Medical Ltd., in
compliance with the European Union Medical Device
Directive MDD93/42/EEC under the supervision of
LRQA Ltd., (Lloyd’s Register Quality Assurance Ltd),
Notifi ed Body number 0088. Verity Medical Ltd., is
certifi ed by LRQA Ltd., to the following Quality
Standards: ISO13485:2016.