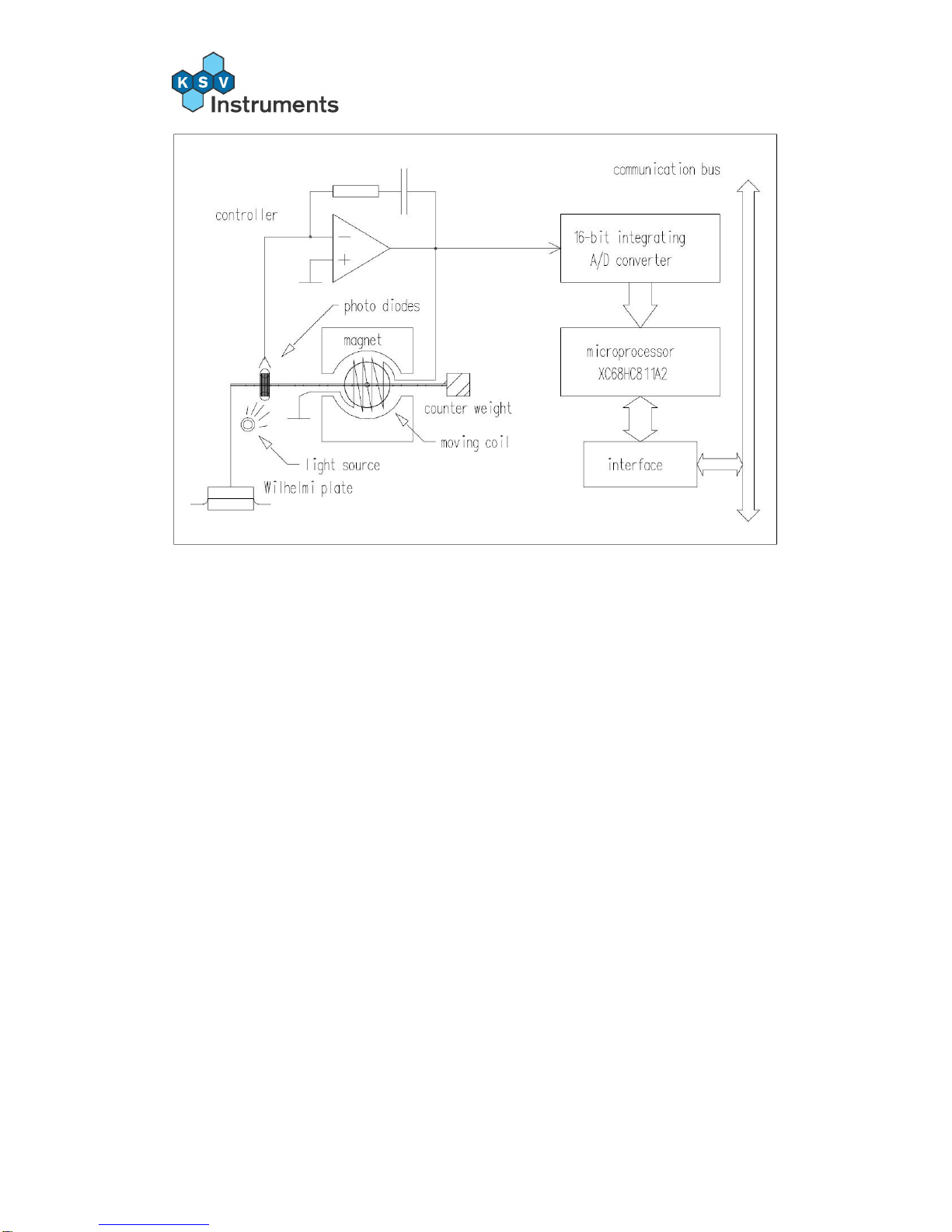
1
1. Introduction
The Langmuir and Langmuir-Blodgett (LB) devices available from KSV
Instruments are efficient and effective in investigating the properties of floating
monolayers, precise deposition of multilayers onto solid substrates or simply
as platforms for use in observing surface chemistry effects such as the
breakdown of an enzyme or the crystalline structure of a surfactant. The wide
range of available systems and the many modules available make
customisation a charm, and if the troughs available do not suit your needs
then contact us about designing a particular trough to your specifications.
There are four series of LB systems available today, the KSV 5000, 2000,
Minitrough and the Minimicro in descending order of size. The differences
between the systems are mechanical, the same software and interface unit
operates all of the LB devices. KSV 2000 is the basic multipurpose LB device.
KSV 5000 adds a sturdy frame with automatic elevator arms for attaching
additional measurement devices, becoming the most versatile LB device
available today. The KSV Minitrough is a more compact and economical LB
device. The KSV Minimicro is a small and elegant LB device brilliant for
depositions on small substrates or to minimise sample volumes.
This is the device-specific manual for the KSV 5000 LB device, featuring
details such as physical descriptions and cleaning instructions. Please see the
LB installation manual or the LB software manual for details on installation,
calibration, measurement taking or data analysis.