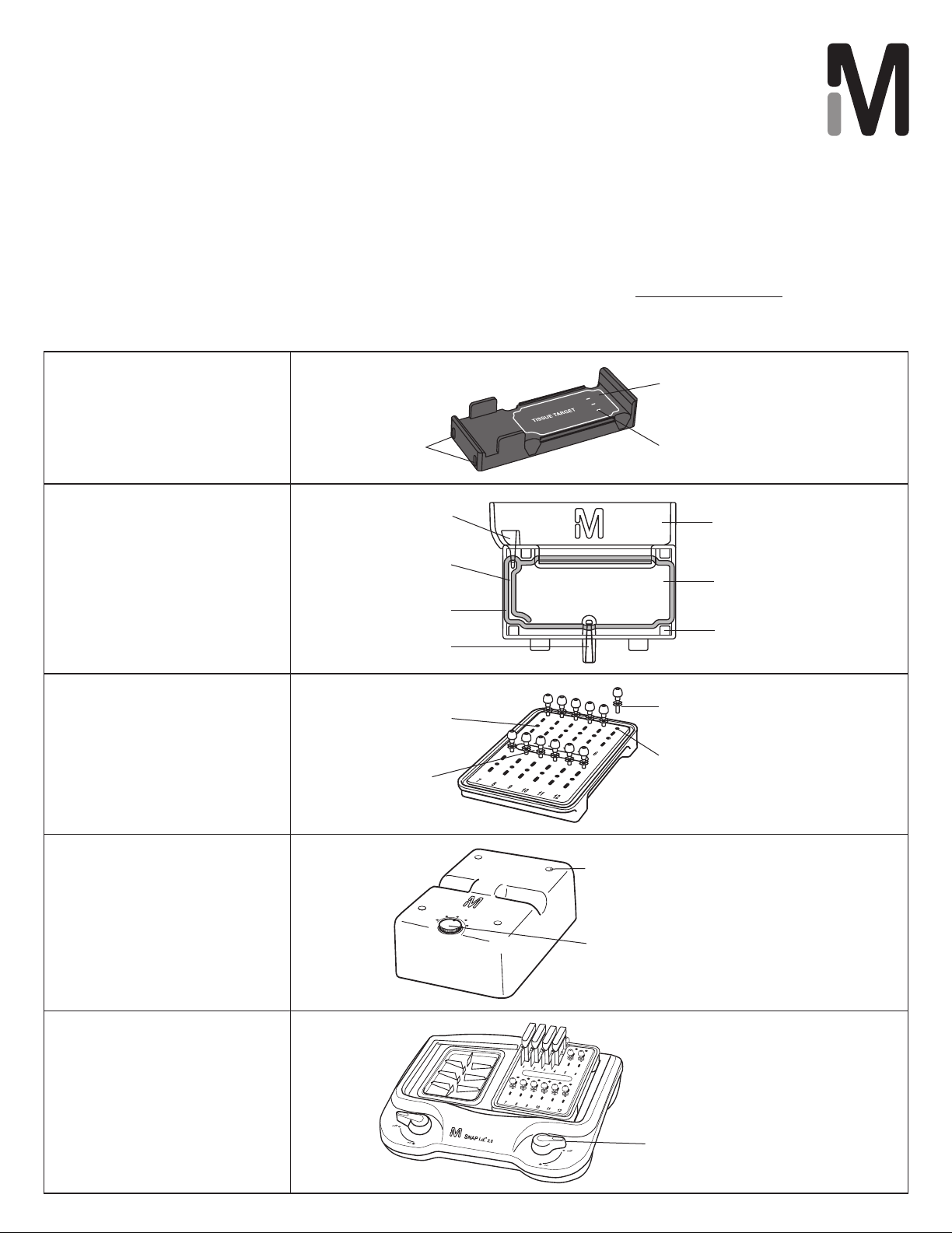
Washing (optional)
1. If washing is required before blocking, fill the wash
reservoir of the slide holder with up to 6 mL of desired
wash solution.
2. To flush the wash solution out of the tissue chamber,
turn on the SNAP i.d.® 2.0 system vacuum.
NOTE: Residual fluid at the bottom of the tissue section
chamber will not impact subsequent steps.
Blocking
1. Load blocking reagent through the injection/recovery
port or the wash reservoir.
2. If incubation is required, place the cover on the IHC
frame and set the task tracker to “blocker”.
linker
primary
blocker
reporter
chromogen
blocker
primary
linker
reporter
chromogen
3. To flush the blocking solution from the slide holder,
remove the cover and activate the vacuum.
4. If wash steps are needed, fill the wash reservoir with
wash solution, then flush by applying vacuum. If
necessary, repeat wash step two more times.
Primary Antibody
1. Inject up to 600 µL of primary antibody through the
injection/recovery port, dispensing slowly to avoid
bubbles, and ensuring complete coverage of the tissue
section.
2. Cover the IHC frame and set the task tracker to
“primary”. When incubation is complete, remove the
cover.
3. Recover primary antibody with a pipette via the
injection/recovery port or flush to waste by applying
vacuum.
4. Fill the wash reservoir with wash solution, then flush
by applying vacuum. Repeat wash step two more times
if required.
Linker (secondary antibody)
1. Inject up to 600 µL of linker through the injection/
recovery port, dispensing slowly to avoid bubbles, and
ensuring complete coverage of the tissue section.
2. Cover the IHC frame and set the task tracker to
“linker”. When incubation is complete, remove
the cover.
3. Recover linker with a pipette via the injection/recovery
port or flush to waste by applying vacuum.
4. Fill the wash reservoir with wash solution, then flush
by applying vacuum. Repeat wash step two more times
if required.
Reporter (tertiary, if required)
1. Inject up to 600 µL of reporter through the injection/
recovery port, dispensing slowly to avoid bubbles, and
ensuring complete coverage of the tissue section.
2. Cover the IHC frame and set the task tracker to
“reporter”. When incubation is complete, remove the
cover.
3. Recover reporter with a pipette via the injection/
recovery port or flush to waste by applying vacuum.
4. Fill the wash reservoir with wash solution, then flush
by applying vacuum. Repeat wash step two more times
if required.
Chromogen
NOTE: Some stains such as hematoxylin and eosin may
permanently stain the SNAP i.d.® 2.0 system.
1. Inject up to 600 µL of desired chromogen through the
injection/recovery port, dispensing slowly to avoid
bubbles, and ensuring complete coverage of the tissue
section.
2. Cover the IHC frame and set the task tracker to
“chromogen”. When incubation is complete, remove
the cover.
3. Recover chromogen with a pipette via the injection/
recovery port or flush to waste by applying vacuum.
4. Fill the wash reservoir with wash solution, then flush
by applying vacuum. Repeat wash two more times if
required.
Slide Removal
1. After staining/counter-staining is complete, a final
wash is recommended before removing the slide from
the slide holder.
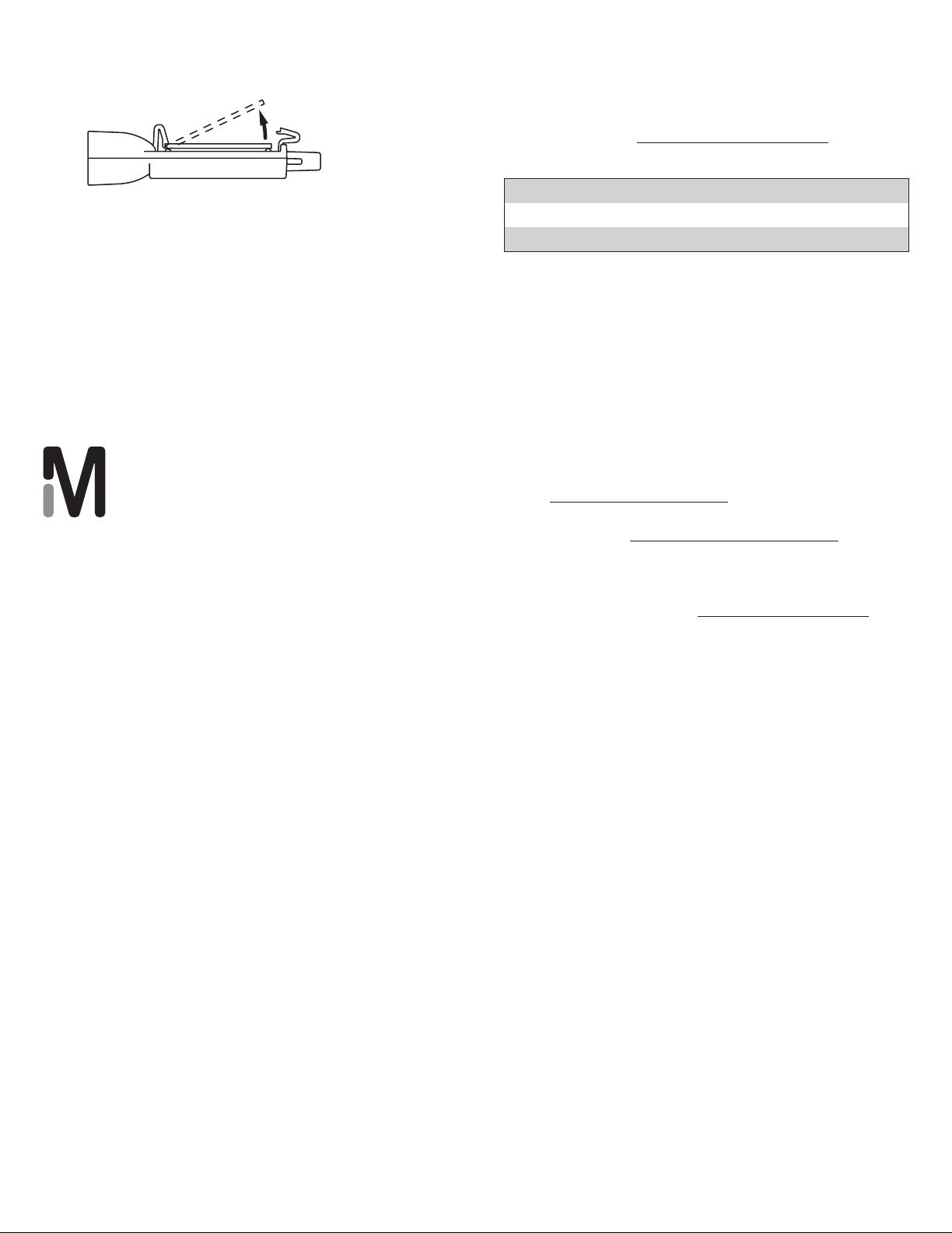
2. To remove the slide, push the assembly fixture
disassembly slot over one of the bottom clips (1).
1
Push slot onto clip
3. Bend the clip 90 degrees away from the slide (2).
Repeat with the other bottom clip.
Bent clip
Bend bottom clips
with assembly
fixture
00119264_RevD.indd 3 1/14/2015 1:26:53 PM