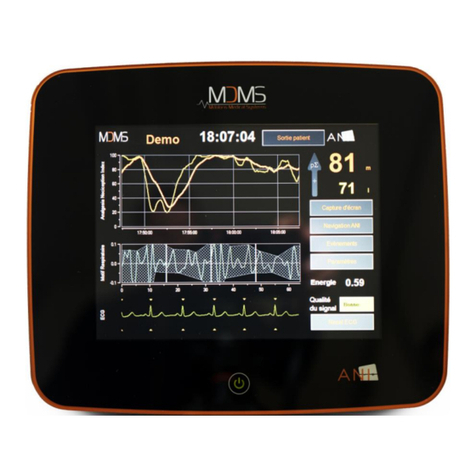
NIPE Monitor V1 - Continuous monitoring system of comfort./.discomfort for newborn infants
MD/PRD/IN16.NIPEV1 V.10 - 12 MAR 2020
Table of contents
1 Safety precautions ........................................................................................................1
1.1 Warnings ..................................................................................................................1
1.2 Caution.....................................................................................................................5
1.3 Notes ........................................................................................................................7
1.4 Key to symbols.........................................................................................................7
2Presentation of the NIPE Monitor V1 ..........................................................................8
3Physiological principle of the NIPE measurement.......................................................9
4Installation of the NIPE Monitor V1 ..........................................................................10
4.1 Clamping support....................................................................................................10
4.2 ECG monitor connexion .........................................................................................10
4.3 Power connector .....................................................................................................11
5. Start the NIPE Monitoring .........................................................................................12
5.1 Set up language.......................................................................................................13
5.2 Demo mode ............................................................................................................13
5.3 Start a new recording ..............................................................................................13
5.4 Resume the previous recording ...............................................................................14
6NIPE display................................................................................................................15
6.1 ECG capture ...........................................................................................................15
6.2 NIPE index.............................................................................................................16
6.3 Display option of the instantaneous NIPE ...............................................................17
6.4 NIPE browsing .......................................................................................................18
6.5 Day / Night Mode...................................................................................................18
6.6 Standby screen........................................................................................................19
7NIPE Monitor V1 settings..........................................................................................20
7.1 Threshold setting ....................................................................................................20
7.2 Insertion and deletion of events...............................................................................21
7.3 Screenshot ..............................................................................................................22
8End of the NIPE Monitoring.......................................................................................23
8.1 Quit the current recording.......................................................................................23
8.2 Maintenance ...........................................................................................................23
8.3 Records management..............................................................................................23
8.3.1 Export data recordings............................................................................................23
8.3.2 Deletion of data recorded........................................................................................25
8.3.3 Setting of data-rates recorded..................................................................................25
8.4 Events update..........................................................................................................26