Mediblink COMPACT M440 User manual

SLO
MEDIBLINK Kompresorski inhalator COMPACT M440
NAVODILA ZA UPORABO
Prosimo, da pred uporabo
izdelka v celoti
preberete navodila za uporabo
HRV
MEDIBLINK Kompresorski inhalator COMPACT M440
UPUTSTVA ZA UPOTREBU
Uputstva su vrlo važna, stoga ih sačuvajte za buduću upotrebu
EN
MEDIBLINK Compressor nebulizer COMPACT M440
INSTRUCTIONS FOR USE
These instructions are important
Please keep them for future reference

EN
Introduction 5
Accessories 6
Device Components 7
General warnings 8
3-stage nebuliser cup 10
2 Masks: for adults and children 12
Mouthpiece 12
Preparing and using the appliance 12
Cleaning and maintaining 13
Checking and replacing lters 14
Technical specications 14
EMC tables 15
Legend of symbols 19
Conditions for storage and transportation 19
Environmental conditions during use 19
Warranty 20
2

SLO
Uvod 22
Dodatki 23
Sestavni deli inhalatorja 24
Splošna opozorila 25
3-stopenjska posodica za ziološko raztopino 27
2 maski: za dojenčke/otroke in za odrasle 29
Ustni nastavek 29
Priprava in uporaba naprave 29
Čiščenje in vzdrževanje 30
Preverjanje in menjava ltrov 31
Tehnični podatki 32
Elektromagnetna odpornost na motnje – EMC tabele 33
Legenda simbolov 37
Pogoji za shranjevanje in prevažanje 37
Okoljski pogoji za uporabo 37
Garancijski pogoji 38
3

HRV
Uvod 40
Pribor 41
Dijelovi uređaja 42
Važna upozorenja 43
Posudica za lijek na tri razine 45
2 maske: za djecu i za odrasle 47
Nastavak za usta 47
Priprema i upotreba otopine za inhalaciju 47
Čišćenje i održavanje 48
Provjera i zamjena ltera 48
Tehnički podatci 49
EMC tablice 50
Simboli 54
Uvjeti čuvanja i transporta 54
Radni uvjeti pri upotrebi uređaja 54
Jamstvo 55
4

Introduction
Dear Customer,
Thank you for choosing Mediblink Compact M440 nebulizer, a piston aerosol
therapy unit designed and manufactured according the most up-to-date
technologies.
Mediblink Compact M440 is a class IIa medical device which converts medicines
from liquid or suspension form to aerosol form, so they can be administered directly
to the airways.
Mediblink Compact M440 nebulizer allows for deeper penetration of the medicine
in the respiratory tract, ensured by the perfect size of the aerosol particles.
Warning!
This product must be assembled, maintained and handled by an adult. Never leave
children alone during use: the unit contains small parts that could be swallowed.
This product must remain in place for use only for the time strictly needed. Once
treatment has been completed, the unit must be stored in a safe place out of reach
of children. Before using the appliance, we highly recommend that you read the
short list of warnings contained in this manual, to make sure you have correctly
understood how to use it.
EN
5
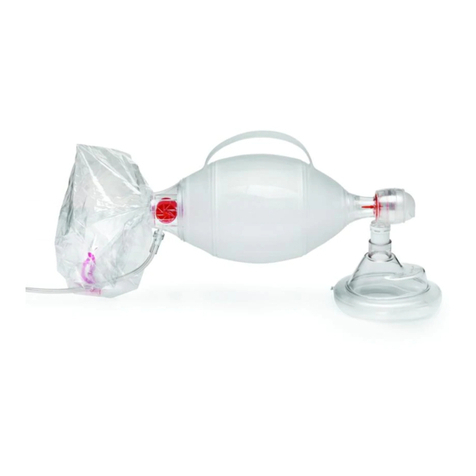
A – Adult mask
B – Children & Baby mask
C – Mouthpiece
D – 3-stage nebulizer cup
E – Connection tube
F – Filters
E
D
A
F
B
C
Accessories
EN
Picture 1: Nebulizer Accessories
6
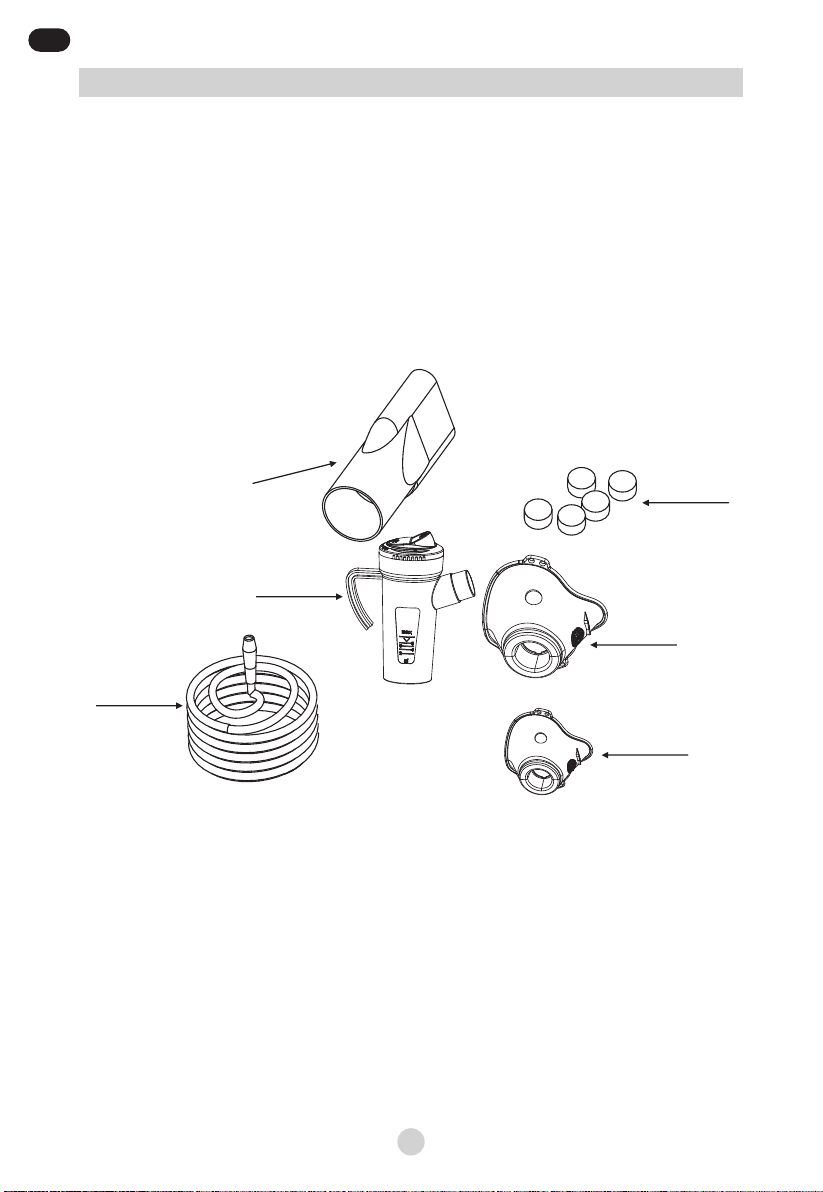
G – On/O switch
H – Compressed air outlet
I – Filter
J – Nebuliser cup support
K – Handle
L – Openings for cooling air
M – Power cord
G
I
KJL
H
M
Device Components
EN
Picture 2: Nebulizer Components
7

1. Before use, carefully read the information in this manual, and keep for future
reference.
2. Unit designed for aerosol therapy. Follow the instructions of a medical
practitioner as to the type of medicine to use, the dose, frequency and duration
of inhalations. Any use other than the one for which the unit is intended is
considered improper and therefore dangerous; Mediblink cannot be held liable
for any damage caused by incorrect or unreasonable use or if the device is
used in electrical systems that do not conform to safety regulations in eect.
3. A power outage, a sudden failure or any other adverse condition may cause
the unit to stop working, therefore it is recommended that users have an
alternative unit or medicine (agreed upon with a physician) that can be used in
the event this occurs.
4. After removing the appliance from its packaging, check that it is intact, without
any visible damage that may have occurred during transport. If in doubt, do not
use the appliance, and contact the Mediblink representative company in your
country.
5 Packaging (bags, box, etc.) may be dangerous and should be kept out of reach
of children.
6 Before connecting the appliance, check that your mains voltage corresponds to
that shown on the rating label. The rating label is located on the bottom of the
appliance.
7 In case of incompatibility between the appliance plug and the electrical socket,
use certied adapters if the existing legislation in the individual country allows
for it or have an Mediblink authorised technician replace the plug.
8 To prevent overheating and damage to the compressor, switch o the
appliance for at least 30 minutes after every 30 minutes of uninterrupted use.
9 Caution! Using the appliance before 30 minutes have elapsed may result in
motor overheating and cause the safety cut-o devices to activate.
10 To ensure correct operation, make sure that the air lter is dry.
11 Do not use the appliance in the presence of nitrous oxide, oxygen or
ammable air/anaesthetic mixtures.
12 Turn o the appliance and disconnect from the mains after use and before
adding more medicine. Do not exceed the maximum level shown on the
nebuliser cup when lling.
13 Keep the appliance and power cord away from hot surfaces.
14 Do not use the appliance while having a bath or shower, or when in a damp
place, or close to bath-tubs, sinks, washbasins, or in any other situation in
which there are liquids that may come into contact with the appliance.
15 Never touch the appliance with wet or damp hands.
General warnings
EN
8

16 Do not drop or put the device into water or other liquids. Should this occur,
unplug it immediately, stop using the appliance and consult a qualied
technician.
17 Do not block the openings for cooling air.
18 Do not use the device if you feel drowsy.
19 If children or persons with physical or mental disabilities use the appliance,
proper supervision is required. Children must be kept under adult supervision
to ensure that they do not play with the appliance. The appliance contains
small parts that can be swallowed and the power cord poses risk of strangling.
20 Do not use connectors or accessories not recommended by the manufacturer.
21 Make sure the appliance is placed on a level, stable surface when used in
order to prevent spillage.
22 During use, the surface must be free of any objects that may obstruct the
correct ow of air.
23 Use the appliance only in vertical position.
24 Never leave the appliance plugged in when it is not in use or when it is
unattended.
25 Before cleaning or performing any maintenance work on the appliance,
disconnect it from the mains by pulling the plug out of the electrical socket.
26 If you decide to no longer use the appliance, disconnect from the mains and
cut o the power cord so the appliance cannot work. Dispose of the appliance
and power cord in compliance with existing laws. We also recommend
eliminating any parts that are potential hazards, particularly for children.
27 Refer to laws in force for information regarding the disposal of accessories
subject to wear. To dispose of the appliance, refer to Directive 2012/19/EU.
28 Do not use the appliance if, after a fall, it shows signs of damage on any of
its parts. If in doubt, do not use the device, and contact the your country‘s
Mediblink representative company Service Centre.
29 In the case of failure or malfunction of the appliance, switch it o by removing
the plug from the mains and contact the Mediblink representative Service
Centre. Do not attempt to open, repair or tamper with the appliance.
General warnings
EN
9
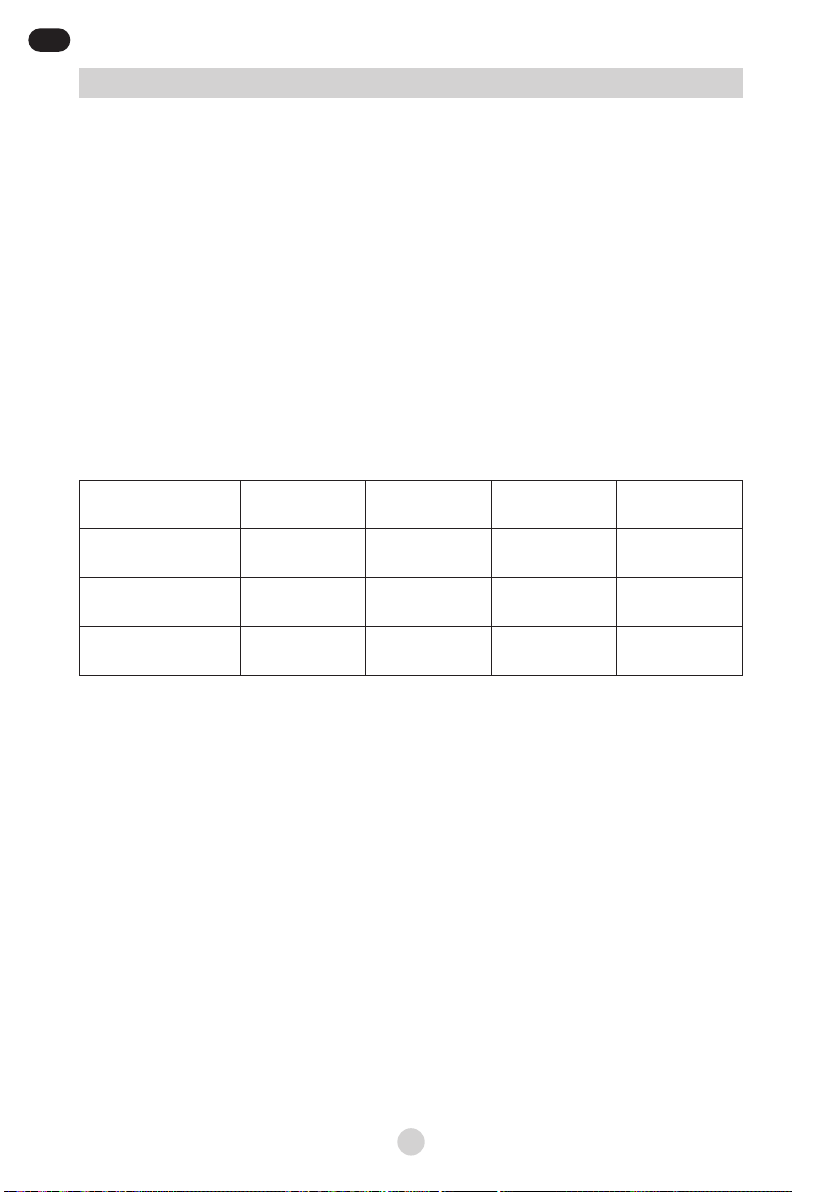
The eectiveness of aerosol therapy depends on the quality of medicine diusion
through the respiratory tract. The nebuliser cup plays an essential role in creating
particles with a diameter that is optimal for therapy, ensuring fast and uniform
treatment.
Mediblink 3-stage nebulizer cup produces particles as small as 3,34 μm (MMAD),
so approximately 78% of particles achieves lower airways, all the way to lungs
alveoli (PORR).
Mediblink 3-stage nebulizer cup also enables the user to set the nebulization rate
to 3 dierent levels: MIN (0,12 ml/min), MED (0,20 ml/min) and MAX (0,30 ml/min).
The nebulization rate (speed) can be changed with a combination of pressing the
BUTTON (options MIN/MAX) on the top of the nebulization cup (Picture 3a) and
switching up and down the blue core, called SPRAY NOZZLE (options MIN/MAX),
located inside the nebulizer cup (Picture 3b).
Table 1: Nebulizer cup nebulization rate settings: MIN / MED / MAX
SPRAY NOZZLE
position MAX MIN MAX MIN
BUTTON on the top
of the cup position MAX MAX MIN MIN
NEBULIZATION
RATE >=0.3ml/min >=0.2ml/min >=0.2ml/min >=0.12ml/min
NEBULIZATION TIME
(5ml of saline solution) 16 min 25 min 25 min 40 min
The button on the top of the nebulizer cup can be switched when the nebulizer
is in use. The switch inside the nebulizer cup must be set to MIN or MAX before
the nebulizer is used and before the saline solution or medicine is put in to the
nebulizer cup.
3-stage nebuliser cup
EN
10
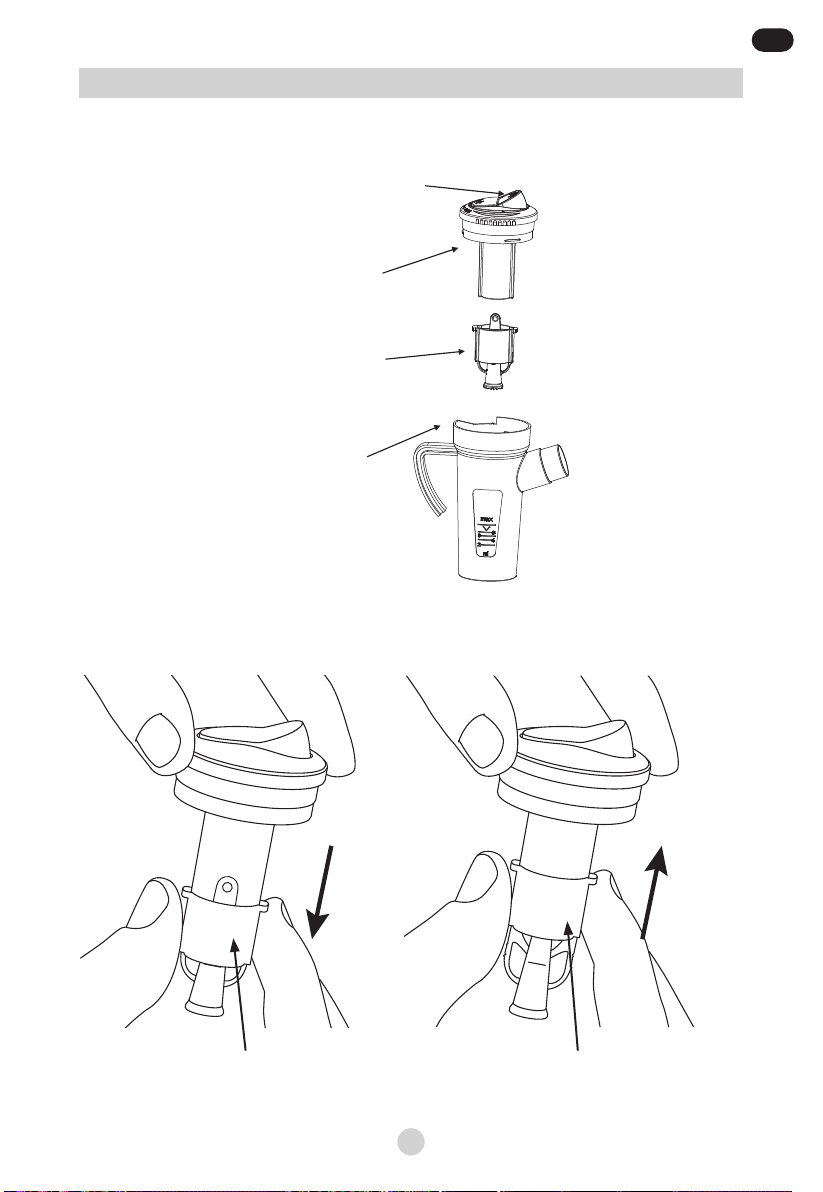
Picture 3a: Nebulizer cup parts
Picture 3b: Nebulizer cup parts
SPRAY NOZZLE
pushed downwards
(MIN nebulization rate)
SPRAY NOZZLE
pushed upward
(MAX nebulization rate)
Cap
BUTTON
(MIN/MAX
nebulization rate)
Spray Nozzle
Medicine Container
3-stage nebulizer cup
EN
MAX
MIN
MIN
11

Soft masks which adapts to your face, minimising drug waste. The masks were
designed to ensure correct and ecient therapy for children and adults alike.
Adults should breath throug the mouth, as this is the most ecient way to deliver
the ow of solution directly into the lower airways, while the children mask should
cover both nose and mouth – this is due to the fact that children are not yet able to
coordinate their breathing.
The mouthpiece can be used alone, without the mask. The mouthpiece conveys
the aerosol jet directly into raspiratory tract. This minimises drug waste while
maximising ecacy.
1. Take the appliance out of the package;
2. Insert the medicine and/or saline solution in the nebuliser cup, according to the
doses recommended by your physician, without exceeding the maximum level;
3. Close the nebuliser cup and take the tube and attach it to the compressed air
outlet on the nebulizer;
4. Connect the tube to the nebuliser cup;
5. Connect the nebuliser cup to the accessory needed for the therapy: mask for
adults and children, or mouthpiece. In case of doubt, ask your physician about
which accessory to use.
6. Connect the power cord plug to the mains;
7. Turn on by pressing the on/o switch and start therapy;
8. Start with nebulization.
9. Carry out treatment preferably seated and in a comfortable position;
10. When the aerosol jet becomes intermittent, interrupt the treatment for a few
seconds to allow the suspended drops of solution to fall o the nebuliser cup
walls. Resume the treatment session, which will be nished when no more
nebulised solution is released from the nebuliser cup;
11. Once the treatment session is completed, switch the appliance o, unplug
it, remove the accessory used and clean the appliance and accessories
according to the instructions in the Cleaning and Maintenance paragraph.
2 Masks: for adults and children
Mouthpiece
Preparing and using the appliance
EN
12

Warning!
Do not wet the device or put it into water or other liquids. For cleaning, use a clean,
dry cloth only.
After each use, remove and detach all the nebulizer components and separately
ush each part (except connection tube) of the nebulizer under running water.
Water temperature must not exceed 50 °C. Allow all nebulizer parts to air-dry
thoroughly.
Do not clean the connection tube (air tube). If the water droplets accidentally enter
the air tube, and the natural wind can not dry the air tube, then only air tube must
be connected to the nebulizer, and the nebulizer must be switched on to discharge
the water droplets from the air tube. When no droplets are left in the air tube, the
nebulizer cup and mask can be attached on the tube, and the user can start using
the nebulizer.
Warning!
Cold disinfect all parts that come in contact with the patient using denatured
alcohol. Carefully dry all components and the device before reassembling. Store in
a cool, dry place, away from light and heat.
Warning!
Never use benzene, thinners or other inammable chemical substances for
cleaning.
For increased hygienic safety, never use the same accessories on more than one
patient. Contact local Mediblink representative to order nebulizer spare parts.
Cleaning and maintenance of the compressor nebulizer
Cleaning and maintenance of the nebuliser cup and accessories
Cleaning and maintenance
EN
13

Periodically check the condition of the lter. The lter was inserted to protect the
compressor. Correct lter maintenance prolongs the life of the nebulizer. The frequency
with which lter should be replaced depends on the conditions in which the unit is used.
The lter is located on the front of the nebulizer.
To replace the lter:
1. Open the lter cover cap;
2. Remove the lter to be replaced from its housing;
3. Carefully t the new lter;
4. Fit the lter cover cap back on.
Warning!
Never leave the lter cover cap and/or lters unattended: These are small parts
posing the risk of suocation if swallowed by children.
Checking and replacing lters
Nominal voltage: 230 V
Frequency: 50 Hz
Power: 200 VA
Fuse: 2,5 A 250 V ~
Maximum ow: 15 ± 2 l/min
Operational ow: 7 ± 1 l/min
Max pressure: 220–300 Kpa
Operational pressure: 11,6–16 Psi / 80–110 Kpa / 0,8–1,1 Bar
Protection against liquid seepage: IPX0
Weight: 1,4 kg
Dimension (L x W x H): 190 x 150 x 108 mm
Conditions for using the device: 30` ON – 30` OFF at 40 °C
MMAD: – aerosol size: 3,34 ± 0,2 μm
PORR (Breathable fraction;
aerosol % smaller then 5 μm): 78,14 % ± 2 %
Nebuliser cup capacity MIN 2 ml; MAX 8 ml
Noise level at 1 m:
65 dB(A); The measurement was made on a new device.
After using the device, the voice level can change.
Nebulization rate: MIN ≥ 0,12 ml/min; MAX. ≥ 0,3 ml/min
Medicine leftover: 0.8 ± 0.05 ml
Medical device classication: IIb
Technical specications
EN
14
EN
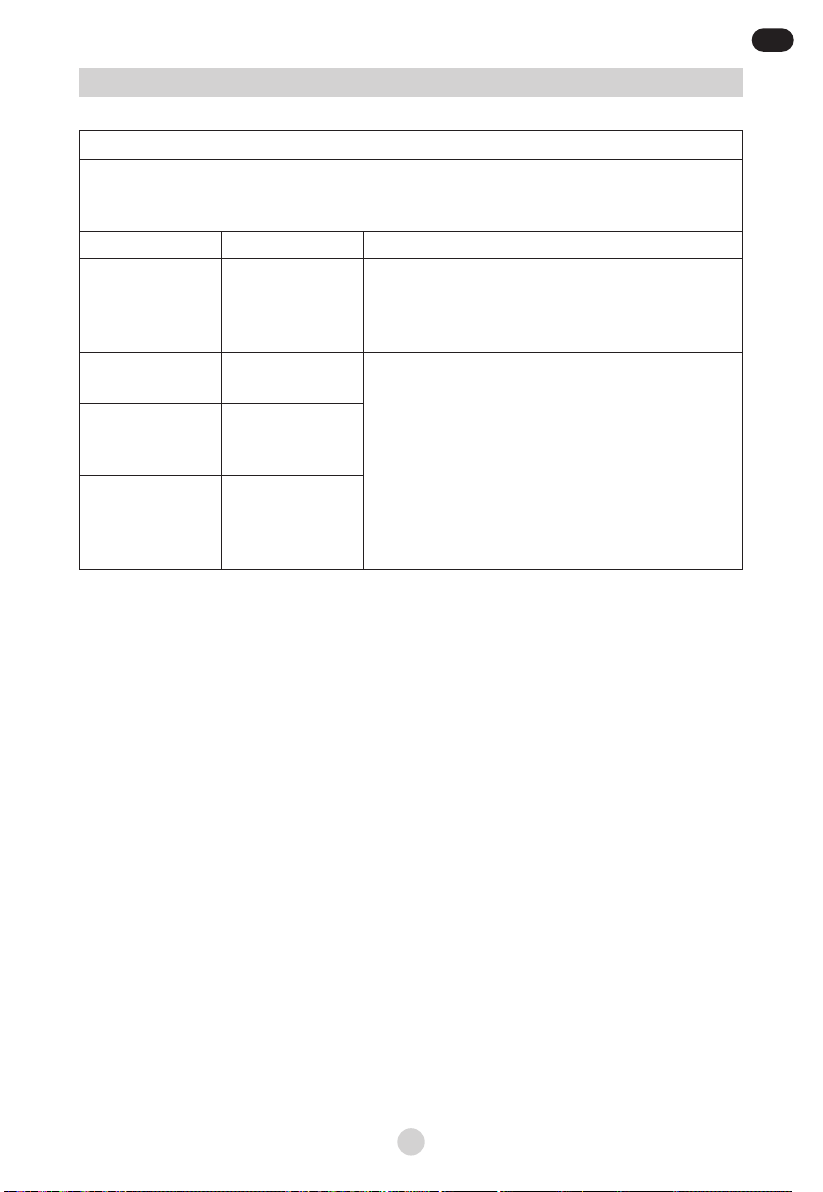
Guidance and manufacturer’s declaration – electromagnetic emissions
The Mediblink Compact Nebulizer M440 (JLN-2313AS) is intended for use in the electromagnetic
environment specied below. The customer or the user of the JLN-2313AS should assure that it is
used in such an environment.
Emissions test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11 Group 1
The JLN-2313AS uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11 Class B
The The JLN-2313AS is suitable for use in all
establishments including domestic establishments
and those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
Harmonic
emissions
IEC 61000-3-2
Class A
Voltage
uctuations/icker
emissions
IEC 61000-3-3
Complies
EMC tables
15
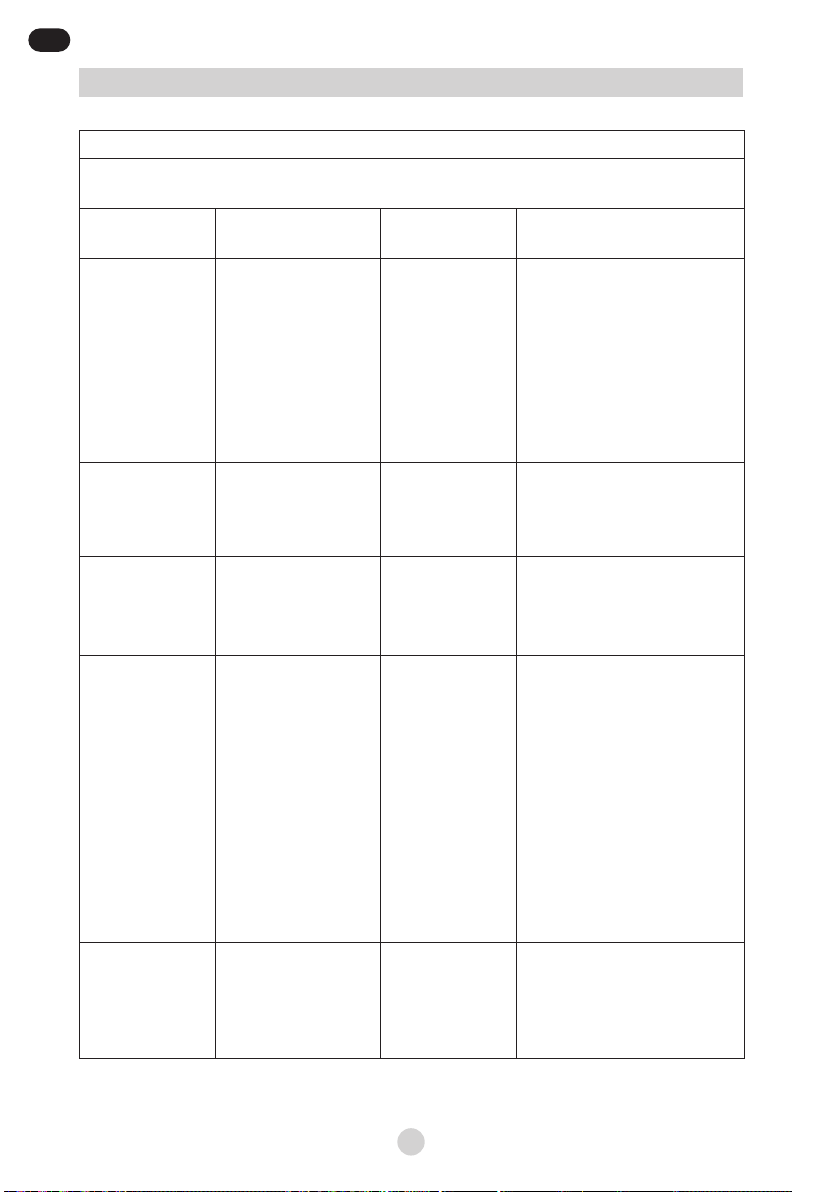
Guidance and manufacturer’s declaration – electromagnetic immunity
TheJLN-2313AS is intended for use in the electromagnetic environment specied below. The
customer or the user of the JLN-2313AS should assure that it is used in such an environment.
Immunity test IEC 60601
test level Compliance level
Electromagnetic environment –
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6kV Contact
±8kV Air
Floors should be wood, concrete
or ceramic tile. If oors are
covered with synthetic material,
the relative humidity should be
at least 30 %. If ESD interfere
with the operation of equipment,
counter measurements such as
wrist strap, grounding shall be
considered.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for input/output
lines
±2 kV for Power
supply lines
Mains power quality should be
that of a typical commercial or
hospital environment.
Surge
IEC 61000-4-5
±1 kV differential
mode
±2 kV common mode
±1kV differential
mode
±2kV common
mode
Mains power quality should be
that of a typical commercial or
hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
<5% UT for
0.5 cycle
40% UT for
5 cycles
70% UT for
25 cycles
<5% UT for
5 s
Mains power quality should be
that of a typical commercial or
hospital environment. If the user
of the JLN-2313AS requires
continued operation during
power mains interruptions, it
is recommended that the JLN-
2313AS be powered from an
uninterruptible power supply or
a battery.
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
3 A/m 3 A/m
Power frequency magnetic
elds should be at levels
characteristic of a typical
location in a typical commercial
or hospital environment.
EMC tables
EN
16
EN
EMC tables
Guidance and manufacturer’s declaration – electromagnetic immunity – for
EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration – electromagnetic immunity
The JLN-2313AS is intended for use in the electromagnetic environment specied below. The
customer or the user of the JLN-2313AS should assure that it is used in such an environment.
Immunity test IEC 60601 test
level
Compliance
level
Electromagnetic environment –
guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2,5 GHz
3 V
3 V/m
Portable and mobile RF communications
equipment should be used no closer to any
part of the JLN-2313AS, including cables,
than the recommended separation distance
calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance
d = 1,2
d = 1,2 80 MHz to 800 MHZ
d = 2,3 800 MHz to 2,5 Ghz
where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in
metres (m).
Field strengths from xed RF transmitters,
as determined by an electromagnetic site
survey, should be less than the compliance
level in each frequency range.
Interference may occur in the vicinity of
equipment marked with the following symbol:
17
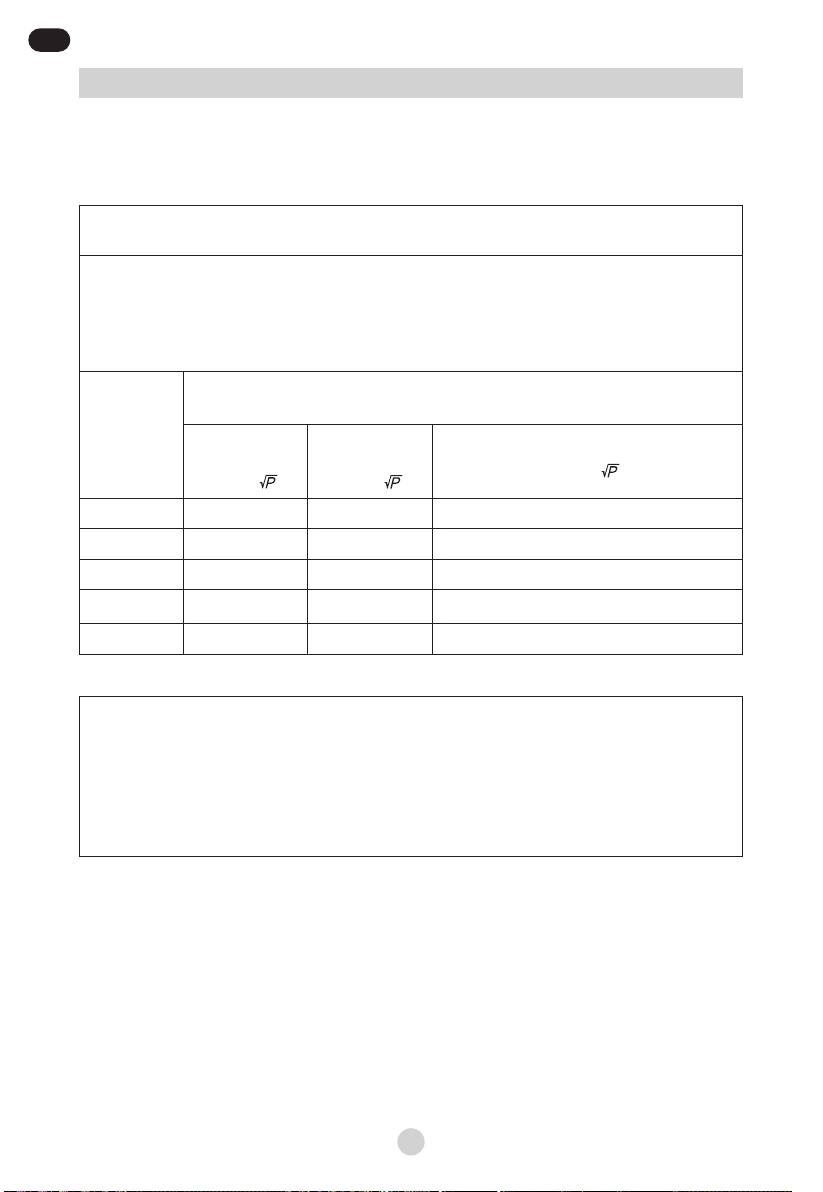
Recommended separation distances between portable and mobile
RF communications equipment and the EQUIPMENT or SYSTEM – for
EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING
Recommended separation distances between Portable and mobile RF communications
equipment and the JLN-2313AS
The JLN-2313AS is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the JLN-2313AS can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the JLN-2313AS as recommended below, according
to the maximum output power of the communications equipment.
Rated
maximum
output power
of transmitter
W
Separation distance according to frequency of transmitter
m
50 kHz
to 80 MHZ
d = 1,2
80 MHz
to 800 MHZ
d = 1,2
800 MHz to 2,5 GHz
d = 1,2
0.01 0,12 0,12 0,23
0.1 0,38 0,38 0,73
11,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
EMC tables
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reection from structures, objects and people.
EN
18

EN
Caution!
Consult instructions for use
Electronic instructions for use:
http://www.mediblink.com/f/m440.pdf
Double insulation
Keep dry (the appliance is not
provided with specic protection
against the penetration of liquids)
Product ID number
Medical device Class IIa
LOT number*
Relative humidity: 30 % / 75 %
Store in a cool, dry place.
Temperature: -10 °C / +55 °C
Device with Type BF applied part
0413
Complies with directive
MDD 93/42/EEC + 2007/47/EC
Alternate current
Serial Number
Number of products in one packaging
Manufacturer
European Union Representative
Conditions for storage and transportation
Legend of symbols
This product complies with directive 2012/19/EU.
The crossed bin symbol on the appliance indicates that the product, at the
end of its life, must be disposed of separately from domestic waste, either
by taking it to a separate waste disposal site for electric and electronic
appliances or by returning it to your dealer when you buy another similar
appliance. The user is responsible for taking the appliance to a special waste
disposal site at the end of its life. If the disused appliance is collected correctly
as separate waste, it can be recycled, treated and disposed of ecologically;
this avoids a negative impact on both the environment and health, and
contributes towards the recycling of the product’s materials. For further
information regarding the waste disposal services available, contact your local
waste disposal agency or the shop where you bought the appliance.
Relative humidity: 45 % / 75 % Temperature: -10 °C / +40 °C
Environmental conditions during use
*Date of manufacture: rst pair of LOT numbers presents the month of manufacture, the second pair of
numbers presents the year of production. Example: LOT 1019 = October, 2019.
19

Warranty
EN
Product: Mediblink Compressor Nebulizer Compcat M440
Manufactured for (also EU importer): Mediblink d.o.o., Gubčeva cesta 19,
8210 Trebnje, Slovenia; [email protected]; www.mediblink.com
Sellers name, address, signature and stamp*:
Date of extradition/sales*:
*If the invoice is accompanied by this warranty, and if all above information can be seen from the invoice, it is not necessary to ll in this eld.
WARRANTY TERMS
Dear customers!
The warranty period is 5 years and starts from the day of product purchase. The
warranty period on spare parts: masks, mouthpiece, tube and lters is 1 year. In case of
product claim, you have to show the invoice. We kindly ask you to save the invoice!
Unfortunately, wrong handling with the device is a reason for 95% of customer
complains. You can easily avoid any problem, by getting useful information provided
by our special service department. To reach our service department, you can call or
send e-mail to Mediblink local distributor.
Before sending the product back to retailer, we kindly ask you to call our service
department, to get help about how to use the device to save you with unneeded trips.
The manufacturer guarantees free elimination of all imperfections due to defects
in material or manufacturing procedure by repairing or replacing the product. In
case that the product can not be repaired or replaced, the customer will get the
money refund. The guarantee is not valid in case of the force majeure, accidents
or unexpected events (such as lightning, water, re etc.), incorrect use or incorrect
transport, non-compliance with safety and maintaining regulations or in case of
unprofessional product intervention.
Traces of every day product usage (scratches, abrasions) and not subject to claim. The
warranty does not eliminate the customer rights, which originate from seller responsibility
for product aws. By accepting the claimed product by the service department, the service
department does not take responsibility for loss of saved data or settings on the product.
All product repairs, which are performed out of product warranty period, have to be paid
by customer by prior notice.
The manufacturer guarantees the product quality and awless product operation in the
warranty period, which starts with the day of product purchase. If the product can not be
repaired in 45 days, the product will be replaced with a new one. In case that the product
can not be replaced, the money will be refunded to the customer.
In case of product claim, call or send e-mail to Mediblink local distributor.
Any serious incident that has occurred in relation to the device should be reported to
the manufacturer (Shenzhen Homed Medical Device CO., Ltd.), company Mediblink
d.o.o. and the competent authority of the Member State in which the user and/or
patient is established.
20
Table of contents
Languages:
Other Mediblink Respiratory Product manuals