Meditech 55B User manual

Page 1of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
INSTRUCTIONS FOR USE
PIN INDEX MEDICAL GAS REGULATOR
PAGE
1. SYMBOLS 2
2. INTENDED USE OF DEVICE 2
3. TECHNICAL DESCRIPTION 3
4. WARNINGS 4
5. INSTRUCTIONS FOR USE 4
6. CLEANING AND DISINFECTION 5
7. INSPECTION AND USER CHECKS 5
8. MAINTENANCE 6
9. ACCESSORIES AND SPARE PARTS 6
10.PERFORMANCE 6
11.SERIAL NUMBERS 6
12.INTENDED LIFE 7
13.APPLICABLE STANDARDS 7
14.MANUFACTURER AND EU REPRESENTATIVE DETAILS 8
READ THESE INSTRUCTIONS PRIOR TO USING THIS EQUIPMENT
A COPY OF THESE INSTRUCTIONS CAN BE FOUND ON OUR WEBSITE (WEB
ADDRESS STATED ON THE BACK PAGE OF THIS BOOKLET)

Page 2of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
CHECK THERE IS NO DAMAGE TO THE DEVICE PRIOR TO OPENING AND USING. IF ANY
DEFECTS FOUND PLEASE NOTIFY THE MANUFACTURER OR AUTHORISED
REPRESENTATIVE.
SYMBOLS
INTENDED USE OF DEVICE
There are two main uses for the Meditech range of Medical Gas Regulators.
Units fitted with a pressure outlet, typically 4 bar, are used to provide gas to a device which
requires an input at this pressure. Primarily, pneumatically powered medical devices such as
a ventilator or Demand Valve. The pressure outlet is supplied in accordance with a national
or international standard and there is the potential for a second pressure outlet for use with
another item. The second outlet may be to the same national or international standard as the
first, or it may be different.
Units fitted with a flow outlet are used to deliver variable flow rates of gas to a patient who
requires gas therapy. The patient will be breathing on his own but may have a need for
support perhaps to supply oxygen enriched air to increase blood oxygen levels. The flow
outlet will be either a ‘fir tree connector’ for use with standard oxygen tubing fitted to a
therapy mask, or canula or a threaded outlet for use with a humidifier. Flow outlets can be
Indicates a potentially hazardous situation which could
result in injury to the user or others, if not avoided.
Read these instructions before using the equipment
Do not use any form of grease or oil
No Smoking
Do not use this device near any source of ignition
Product part number
Serial Number
Manufactured by
Date of manufacture and country of manufacturer.
Next Maintenance or Service Due date
Storage and operating temperatures.
Authorised Representative details
Medical Device

Page 3of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
switched between different rate in the range of 0.5lpm (litres per minute) to 15 lpm, with an
optional ‘MAX’ setting of approximately 25lpm used for system purging.
Units are available with both a pressure outlet and a flow outlet and these can be used for
either purpose or both simultaneously.
TECHNICAL DESCRIPTION
This manual refers to B.N.O.S. Meditech 55B and 55SL medical gas regulators with pin index
input fittings.
55B Range regulators are designed for mobile use in demanding conditions. The main material of
manufacture is brass. They are also suitable for use in static conditions.
55SL Range regulators are designed for mobile use where light
weight is important. The main material of manufacture is aluminium
alloy, but with a brass high pressure Medical Gas path for safety. They are also suitable for use
in static conditions.
Figure A: Pin Indexed Regulator.
Key to components
a. Regulator body
b. Pressure gauge
c. Yoke
d. Yoke Screw
e. Yoke Sealing Washer
f. Pin Index
g. Output Quick Connection
h. Probe Quick Connection
i. Therapy Mask Elastomeric Connection
j. Therapy Outlet from Regulator
k. Therapy Outlet Flow Selector
l. Therapy Outlet Flow Display Window
Figure B: Pin Indexed Regulator assembled to Medical Gas Cylinder
Key to components
a. Regulator body
b. Pressure gauge
c. Medical Gas Cylinder Valve
d. Yoke
e. Yoke Screw
f. Medical Gas Cylinder Valve Body
g. Medical Gas Cylinder
h. Probe Quick Connection
i. Output Quick Connection
j. Therapy Mask Elastomeric Connection
k. Therapy Outlet from Regulator
l. Therapy Outlet Flow Selector
m. Therapy Outlet Flow Display Window

Page 4of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
WARNINGS!
THIS MEDICAL GAS REGULATOR IS TO BE USED BY ADEQUATELY TRAINED PERSONNEL
ONLY.
The user should ensure that no grease, oil or other contaminants come into contact with the
cylinder, valve, regulator, gauge(s), flow selector or connections to mitigate risk of fire.
Do not use this device near any source of ignition e.g., naked flame, electrically powered
heaters, cigarettes etc.
Always open the cylinder valve slowly to minimise pressure shocks.
The connectors fitted to the Regulator are designed specifically for this device. If
replacements are needed, they must be approved parts supplied by B.N.O.S. Meditech Ltd.
The Regulator must not be disassembled when it is under pressure, as serious injury could
result.
The Therapy Outlet Flow Selector (if fitted) must be set at the defined settings shown on the
dial. It must not be set between adjacent settings, as this may result in no flow from the
outlet.
The accuracy of the Therapy Outlet Flow selector (if fitted) will be affected if the input
pressure is varied from the nominal value shown in the Performance section of this
document.
Note that increasing output flow is obtained from the Therapy Outlet Flow selector (if fitted) if
the control is turned clockwise.
The setting of the Therapy Outlet Flow selector (if fitted) does not indicate that a flow is
occurring. The User must check that medical gas is flowing to the patient by another means.
Attention is drawn to the accuracies stated in the Performance section of this document.
4.11 Do not use the flow outlet for driving any medical equipment.
4.12 The use of the regulator for gases other than that on the device labelling is prohibited.
4.13 Do not attempt to modify the fittings to suit other gases or fitting systems.
INSTRUCTIONS FOR USE
(EXAMPLE IN PICTURE IS SHOWING A BS SCHRADER (BS 5682) TYPE. THIS MAY VARY IF USING
INTERNATIONAL FITTINGS)
5.1. ATTACHING TO A CYLINDER
5.1.1 Inspect the Pin Indexed Regulator (Fig: A) and check that the Yoke sealing washer (e) is in
place and in serviceable condition.
5.1.2 Check your Medical Gas Cylinder to ensure that it is correctly labelled and the correct
Medical Gas. Ensure that any new gas cylinders are fitted with a tamper evident seal.
Remove all traces of the seal. The Index Pins (f) prevent incorrect attachment of the wrong
type of gas cylinder and will locate only into the two matching holes on the face of the
corresponding type of Medical Gas cylinder valve.
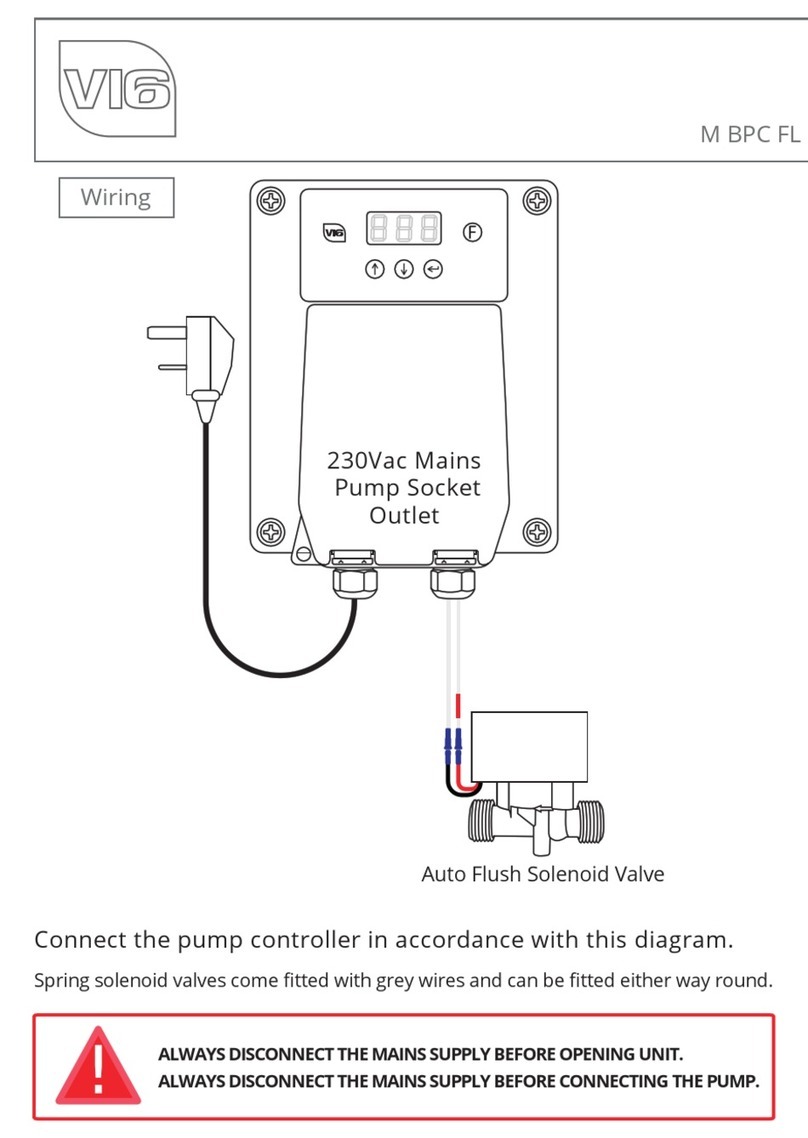
5.1.3 Position the Regulator Yoke (Fig: B) (c) over the Cylinder valve body (n), having first
loosened the Yoke Screw (d). Position the Index Pins (f) into their locating holes and tighten
Yoke Screw (d) securely, ensuring that the Pressure Gauge (b) and Outlets (g and j) are in
the correct orientation. We recommend the orientation shown in Figure B where the output
quick connection (g) is close to the Medical Gas cylinder body (m). During assembly ensure
the regulator gauge is not compressed against the cylinder valve, which can damage the
gauge and prevent the Yoke sealing washer functioning. When the assembled regulator and
cylinder is placed in the kit bag, the cylinder gauge (b) and therapy outlet flow display
window (l) can be easily seen and the cylinder valve key (o), output quick connection (g)
and Therapy outlet flow selector operated.
5.1.4 Open the Cylinder Valve (k) slowly (i.e. facing away from the operator or other personnel)
by means of the Cylinder Valve Control (o). If gas escapes around the Yoke sealing washer,
turn off cylinder with the Cylinder Valve Control (o) and tighten the Yoke Screw (d). Again

Page 5of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
slowly open the cylinder valve, if no gas leak is heard, open the Cylinder Valve one full turn
and check that the Pressure Gauge (b) shows that the cylinder is full.
5.1.5 If a leak of gas is still heard, turn off the Cylinder Valve and remove the Regulator. Remove
and replace the Yoke sealing washer (Fig: A) (e) and repeat from step 5.1.1.
5.1.6 To attach a Medical Gas-powered device to the Regulator, insert male Probe (fig A) (h)
which is attached to the supply hose into the female Outlet (g) by pushing firmly into the
orifice. The coupling will lock automatically.
5.1.7 To attach equipment to the therapy outlet (j), connect with elastomeric tubing. When
connected select the required flow rate by rotating the Therapy outlet flow selector (k) and
observing the flow rate selected in the Therapy outlet flow display window (l).
5.1.8 After use the cylinder valve should be turned off.
5.1.9 The Therapy outlet flow selector (k) should always be left in the off position (“0” indicated in
the Therapy outlet flow selector window (l)) when the therapy outlet is not in use. This
prevents Medical Gas escaping un-noticed from the outlet (j) when the Medical Gas bottle is
turned on.
5.2. TO CHANGE A USED CYLINDER
5.2.1 Turn off the Cylinder Valve (Fig: B) by the Cylinder Valve Control (o)
5.2.2 De-pressurise the regulator by operating the therapy flow or equipment connected to the
Outlet (e). The pressure gauge (b) reading will move to zero. Turn the therapy flow to the off
position (“0” indicated in the Therapy outlet flow selector window (l)) Uncouple the Probe by
twisting the knurled collar on the female Outlet (e) in a clockwise direction IF using a BS
Schrader outlet. The male probe will automatically disconnect.
NOTE: If another outlet type of international fitting is used this may require another
force (e.g., pull) different to the BS Schrader of twisting clockwise to disconnect.
5.2.3 Unscrew the Regulator Yoke Screw (d) and remove the empty Cylinder ensuring the Yoke
sealing washer (Fig: B) (e) remains in place on the regulator.
5.2.4 Repeat the instructions for "Attaching to a cylinder”. (Section 5.1)
CLEANING AND DISINFECTION
6.1 Surface cleaning of the regulator should be carried out using soap flakes in solution when
disconnected from the gas supply. Never immerse the regulator in any fluids.
6.2 For disinfection purposes a chlorine dioxide-based product (e.g. the Tristel Wipes System)
should be used, at a nominal concentration of 0.02% wt./vol. The concentration refers to
chlorine dioxide in water. The regulator should be wiped clean only and should not be
submerged in any fluids.
6.3 After cleaning or disinfection, the regulator should be wiped with clean water to remove
any residue and then allowed to dry before returning to use.
NOTE: Pressure regulators are not suitable for autoclaving.
INSPECTION AND USER CHECKS
7.1 INSPECTION
7.1.1 The regulator should be inspected for damaged or broken components and contamination
after each use. Check that the Pressure Gauge (b), Power Output (g) and Therapy Outlet (j)
(if fitted) are all tight and undamaged. Check that any controls operate correctly and that the
Yoke Screw (d) operates smoothly.
7.1.2 If contaminated, the Regulator should be cleaned in accordance with Section 6.
7.1.3 If damaged, the Regulator should be withdrawn from service and returned to B.N.O.S.
Meditech for assessment and repair.
7.2 USER CHECKS (to be carried out before and after cleaning). Ensure no blockages of the
pressure relief valves (frozen in extreme temperature)
7.2.1 Connect the regulator to a suitable Medical Gas cylinder. Check the pressure gauge (b)
reads zero. Slowly open the cylinder valve. Check the pressure gauge indicates that there is
pressure in the cylinder. If it continues to read zero, use a cylinder known to contain gas at a
minimum pressure of 50 bar.

Page 6of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
7.2.2 Check for audible leaks when the therapy outlet flow selector (k), if fitted , is in the off position
(0 indicated in the therapy outlet flow selector window (l)).
7.2.3 For regulators with a Therapy Outlet Flow Selector, check that the operation of the selector (k)
has distinct stops at all settings but can be operated smoothly between settings without undue
force. Check that the Medical Gas output increases progressively as the control is operated
from 0 to 15 (or MAX). Check there is no flow when the selector is returned to 0.
7.2.4 Connect an appliance (e.g. a resuscitator) to the Power Output (g). Check that the appliance
is capable of being operated from the Power Output (i.e. Medical Gas is being supplied to it)
and that there is no audible leakage from the connector. Disconnect the appliance. Check that
the connector operates correctly during the connection and disconnection process.
7.2.5 Close the cylinder valve.
7.2.6 If the Regulator fails any of these checks, it should be withdrawn from service and returned to
B.N.O.S. Meditech Ltd. for assessment and repair.
8. MAINTENANCE
8.1 Maintenance must be carried out on the unit on a five-yearly basis by B.N.O.S. Meditech
or engineers certified by B.N.O.S. Meditech. This activity involves dismantling the unit
and replacing all internal seals and any components which show significant wear and
tear.
8.2 Performance should also be checked on a five-yearly basis, using suitable test
equipment. The dynamic and static regulator pressure should be measured at the Power
Output, using a Medical Gas cylinder with a minimum content of 75 bar. Verify that the
flow rates on all settings are within specification at room temperature. If required,
B.N.O.S. Meditech Ltd. can advise on suitable test equipment.
8.3 A leak test should also be performed by applying a Medical Gas compatible leak test
solution to all outlets, fittings and joints, where applicable.
8.4 B.N.O.S. Meditech Ltd. offers training and certification on the service, repair and
preventative maintenance of Meditech products.
9. ACCESSORIES AND SPARE PARTS
No specific accessories are listed for the Regulator. Spare parts that are replaceable by the end
user are as follows:
Protective boot for pressure gauge 633-0027-00
Yoke sealing washer (also known as bodock seal) 033-1021-00
Additional parts are available to medical engineers. Contact the manufacturers for details.
10. PERFORMANCE
Rated maximum upstream pressure (P1): 200 bar
Rated outlet pressure (P2): 4 bar (7 bar variant available for Medical Air)
Test inlet pressure (P3): 8 bar
Standard discharge (Q1): 50 lpm (litres per minute)
Variation of outlet pressure (P2) when inlet pressure is varied from P1 to P3 at a flow of Q1: 4%
For regulators with a Therapy Outlet Flow Selector, the figures represent the flow rate from the
“Therapy outlet” in litres per minute (lpm). These figures are subject to a tolerance of ±20% of
each stated value or ± 30% of each stated value for flows of 1,5l/min or less. If the Therapy Outlet
Flow Selector has a MAX setting, this has a nominal flow rate of 25 lpm.
Temperature ranges: Storage: -40°C to +60°C
Operating: -20°C to +60°C
11. SERIAL NUMBER
The Serial No. is to be found on the “barrel” label of the regulator. It consists of four sections the
first letters expressing the general type of device, followed by numbers for the month and year of

Page 7of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
manufacture and lastly a set of up to five numbers representing the “number” of the unit and
differentiating units built in the same month.
Example for Therapy Regulators:
RT 03 21 12345
Regulator Therapy Month of Manufacture Year of Manufacture Number of Unit
Example for Non-Therapy Regulators:
R 03 21 12345
Regulator Month of Manufacture Year of Manufacture Number of Unit
12. INTENDED LIFE
This Regulator has been designed for the demands of the pre-hospital emergency medical market
to give many years of reliable service. The regulator is manufactured from the finest quality
materials with individual components subject to strict quality control tests to ensure high standards
under ISO 13485. The regulator is designed to have a product life span of 10 years, excluding
abuse to the instrument.
Disposal should follow the healthcare setting guidelines.
We reserve the right to change design without prior notice.
13. APPLICABLE STANDARDS
B.N.O.S. Meditech Ltd. is an ISO 13485:2016 certified company.
B.N.O.S. Meditech Regulators are supplied in conformity under a quality system to meet
Medical Devices Directive 93/42/EEC
Regulators are classified as Class IIb Medical Devices.
The above quality system has been inspected by the Notified Body Ref: CE 2797 being BSI,
Say Building, John M. Keynesplein 9, 1066 EP Amsterdam, The Netherlands.
The following National & International Standards apply to the device:
Standard Number:
Title:
BS EN ISO 10524-1:2019
Pressure regulators for use with medical gases
– Part 1: Pressure regulators and pressure
regulators with flow-metering devices
BS 5682:2015 OR International Fittings (if
applicable)
Specification for probes (quick connectors) for
use with medical gas pipeline systems
BS EN ISO 407:2021
Small medical gas cylinders-Pin index yoke-
type valve connections
BS EN ISO 15001:2011
Anaesthetic and respiratory equipment.
Compatibility with oxygen
BS EN ISO 15223-1:2021
Medical devices – Symbols to be used with
medical device labels, labelling and information
to be supplied
EN ISO 20417:2021
Medical devices. Information to be supplied by
the manufacturer.
It is also certified that the equipment listed above fully complies with all the required mandatory
standards and the performance, specifications, standards and sources agreed and contracted for
this order.

Page 8of 8
111-0058-00 (Doc 030M) 23/05/2022 ISSUE V
IMPORTANT NOTICE
Manufacturer’s Warranty is for a period of 5 years and includes parts and labour. It does not include
transport costs. The responsibility and cost of returning and collecting the unit from the manufacturer
or their authorised representative is the owners.
Any disassembly of the regulator beyond that detailed in this manual will invalidate the
warranty and the manufacturers disclaim any liability for products that have undergone
unauthorised repair.
COMPANY CONTACT DETAILS
This regulator is designed and manufactured by:
B.N.O.S. Meditech Ltd.
9 Fifth Avenue, Bluebridge Ind. Est.,
Halstead, Essex CO9 2SZ, England
Tel: +44 (0)1787 479475,
Fax: +44 (0)1787 477747
E-mail: sales@meditech.uk.com
www.meditech.uk.com
EU REPRESENTATIVE:
MEDICAL DEVICE MANAGEMENT LTD
BLOCK B, THE CRESCENT BUILDING
NORTHWOOD, SANTRY
DUBLIN 9, D09 C6X8
IRELAND
2797
This manual suits for next models
1
Table of contents
Popular Controllers manuals by other brands

Telwin
Telwin START PLUS 4824 instruction manual
Cooper Power Systems
Cooper Power Systems Kyle Type ME Series Maintenance instructions

Datakom
Datakom DKG-109 user manual

Adaptec
Adaptec 2930U2 - Storage Controller Ultra2 SCSI 80... User reference

Prolon
Prolon NC2000 Hardware guide

Rinnai
Rinnai MC-601-W Operation & installation manual