Medonic CA620 User manual

User´s Manual
Medonic CA620 + MPA + AD
Medonic CA620 + MPA
Medonic CA620
Medonic CA530 + MPA + AD
Medonic CA530 + MPA
Medonic CA530
Medonic CA620-CellGuard + MPA + AD
Medonic CA620-CellGuard + MPA


03-11-24 3
Contents
Preface ............................................................ 7
1 Safety Instruction........................................9
1.1 Intended Use .......................................................................... 9
1.2 Counter Indications ............................................................... 9
1.3 Warranty Limitations.............................................................. 9
1.4 General Warnings .................................................................10
1.5 Emergency Procedure .........................................................11
1.6 Warning Signs in Manual.......................................................11
1.7 Signs on Equipment .............................................................12
2 Specifications............................................ 13
2.1 General ...................................................................................13
2.2 Description of Available Parameters..................................13
2.3 Short-List of Specifications..................................................14
2.4 Parameter Ranges ...............................................................16
2.5 Reagents and Reagent Consumption .............................. 17
2.6 Limitations ..............................................................................18
2.7 Differences between CA620 and CA530
(9,16,20 parameters)........................................................... 22
3 Measuring Principles ............................... 25
3.1 RBC,WBC and PLT Concentration Detection ................ 25
3.2 Sizing RBC, WBC and PLT ................................................. 27
3.3 Counting Time RBC & WBC ..............................................28
3.4 WBC Differentials ................................................................. 28
3.5 MCV (Mean Cell Volume RBCs)........................................ 30
3.6 RDW% (Red Cell Distribution Width)................................. 30
3.7 RDWa (Red Cell Distribution Width Absolute) ................. 30
3.8 HCT (Hematocrit)................................................................. 30
3.9 PLT (Platelets)....................................................................... 30
3.10 MPV (Mean Platelet Volume)..............................................31
3.11 PDW (Platelet distribution width)........................................31
3.12 LPCR (Large Platelets)........................................................ 32
3.13 PCT (Packed Platelet Volume) .......................................... 32
3.14 HGB (Hemoglobin Concentration) ................................... 32
3.15 MCH (Mean Cell Hemoglobin) .......................................... 32
3.16 MCHC (Mean Cell Hemoglobin Concentration)............. 32
4 Parameter Flags....................................... 33
4.1 TU (Time-out Upper Detector) .......................................... 34
4.2 TL (Time-out Lower Detector)........................................... 34
4.3 SE (Statistical Error) ............................................................. 34
4.4 DE (Distribution Error).......................................................... 34
4.5 FD (Floating Discriminator) ................................................. 35
4.6 OF (Offset error HGB) ......................................................... 35
4.7 LO (Low blank level HGB)................................................... 35
4.8 HI (High blank level HGB).................................................... 35
4.9 NG (Negative HGB) ............................................................. 35
4.10 SE (Statistical error HGB).................................................... 36
4.11 TB (Air bubbles).................................................................... 36

4 03-11-24
4.12 NM, OM, TM, BD (WBC Differential Flags) .......................36
4.13 RP (Red Cells Present)........................................................36
4.14 EC (Expired control).............................................................37
4.15 Comments on Flagging Capabilities .................................37
5 Installation .................................................39
5.1 Unpacking the Instrument ..................................................39
5.2 Working Conditions .............................................................39
5.3 Mechanical Check and Set-Up.......................................... 41
5.4 Front Panel and Command Keys......................................44
5.5 Filling the system with reagents.........................................46
6 Initial System Configuration......................47
6.1 CA620-CellGuard ................................................................47
6.2 Setting up the CA620-CellGuard
for Pre-Donor Screening.....................................................47
6.3 Setting up the CA620-CellGuard
for PLT Concentrate ............................................................48
6.4 CA620/530 ...........................................................................48
6.5 Setting Date and Time.........................................................49
6.6 Printer Configuration............................................................49
6.7 Select Language .................................................................. 51
6.8 Select Units ........................................................................... 51
7 Routine Operation .....................................53
7.1 Background Check..............................................................53
7.2 Analysing the Sample (Open Tube) ..................................54
7.3 Analyzing the Sample (Pre-Dilute).....................................56
7.4 Analysing the Sample (Micro Pipette Adapter, MPA).....57
7.5 Analysing the Sample (Cap Piercing Device) ..................58
7.6 Advanced User Options (CA620-CellGuard) ..................60
8 User Interface ........................................... 61
8.1 Sample Memory................................................................... 61
8.2 Setup Menu...........................................................................64
8.3 Setup Menu 2 .......................................................................68
9 Warning Displays ......................................75
9.1 Warnings Related to the MPA and Pre-Dilute Inlet.........75
9.2 Stand-By Mode ....................................................................76
9.3 Reagent Empty .................................................................... 77
9.4 Printer and Serial Output .....................................................78
9.5 Auxiliary Warnings/Messages ............................................79
10 Calibration and controls ........................... 81
10.1 Introduction ........................................................................... 81
10.2 Use of Calibrators and Controls ........................................ 81
10.3 Calibration on a Known Sample ........................................82
10.4 Calibration Through a Certified Calibrator........................83
10.5 Calibration on Pre-Diluted Capillary Blood .......................85
10.6 Calibration of the MPA.........................................................85
10.7 Calibration of RDW, LYMF and GRAN ..............................86

03-11-24 5
11 QC and Blood Controls .............................87
11.1 Introduction........................................................................... 87
11.2 Initializing the Levey-Jennings Plots and Functions ....... 87
11.3 Use of Blood Controls and Levey-Jennings Plots ......... 88
11.4 Initialization and Use of X-B Function................................ 89
11.5 Display of Blood Control Data.............................................91
12 Printer and Serial Output ......................... 93
12.1 Selecting the Correct Printer Type ................................... 93
12.2 The Serial Output/Hardware Connections ...................... 93
12.3 The Serial Output Format ................................................... 94
13 Maintenance, Shut-Down & Transport.... 95
13.1 Daily Maintenance ............................................................... 95
13.2 Monthly Maintenance ......................................................... 95
13.3 Three (3) Month Maintenance........................................... 95
13.4 Short Term Transport.......................................................... 96
13.5 Re-packaging and Long Term Transport........................ 96
13.6 Permanent Shut-Down and Storing the Instrument...... 97
13.7 Disposal information ............................................................ 97
14 Trouble Shooting...................................... 99
14.1 Counting Time HI (TU & TL) ............................................... 99
14.2 Counting Time LO................................................................ 99
14.3 HGB Value Flagged with LO............................................... 99
14.4 HGB Value Flagged with HI .............................................. 100
14.5 HGB Value Flagged with OF ............................................ 100
14.6 High Background PLT....................................................... 100
14.7 Indication Number XXX Displayed.................................... 101
14.8 Cell Diff. Only on a Few Samples..................................... 102
14.9 Cap Piercing Device .......................................................... 102
14.10Clogging in Aspiration Tube ............................................. 104
Index............................................................ 105

6 03-11-24

03-11-24 7
Preface
Name of product, serial number and software
version
This manual describes:
CA620, CA530 and CA620-CellGuard as well as the optional devices “Closed
Tube Adapter” (Cap Percing Device) and MPA (Micro Pipette Adapter)
Serial number of the instrument is found on the serial plate on the rear of the in-
strument. Software version is displayed on the main menu in the upper right cor-
ner of the display, see picture below marked “A”
Other documentation related to this manual:
Additional applications and notes, as well as service manual, product data sheets
on reagents and calibrators are available from your local distributor and listed on
the Boule support server, which is exclusively available to your authorized distrib-
utor.
Specimen, special conditions of collection and
storage conditions
General instructions for (human) blood collection, handling, storage of samples
etc. are described in the booklet “Hematology for sales and service engineers”
which is available from your authorized distributor.
Operator training
Additonal operator training is not required under the following conditions:
1. The operator must have basic scills in working under laboratory conditions.
2. The operator must have basic scills in hematology.
3. The operator must be aware of IVD(EU) and FDA(USA) requirements re-
garding laboratory equipment for In Vitro Diagnostic purposes.
4. The operator must read and understand this manual.
Component lists, tools and other consumables
These are listed, including their special functions, in the Service Manual distrib-
uted on CD-ROM and available via the Interrnet ‘on-line’ to your authorized dis-
tributor.
1177en.gif
A
1176en01

8 03-11-24 1176en01
Name and address of manufacturer:
Boule Medical AB
P.O. Box 42056
SE-126 13 Stockholm
Sweden
Telephone: +46 8 744 77 00
Fax: +46 8 744 77 20
e-mail: [email protected].se
Name and address of distributor
Distributors are listed on:
http.//www.boule.se
Standards
EN591:2001
IVD 98/79/EG
SSEN 61010-2-101 (Low Voltage 73/23/EEC)
EN 61326 (1997) with amendment EN 61326/A1 (1998) (EMC 89/336/EEC)
Standards harmonized with FDA/UL
Manual number
1504062en
Date of issue
August 2003

03-11-24 9
1 Safety Instruction
1.1 Intended Use
CA620/530 and CA620-CellGuard
The CA620/530 is a fully automated hematology analyser used for in vitro diag-
nostic testing of whole blood specimens under laboratory conditions. The user
must have basic skills in laboratory practice and must be aware of the guidelines
in “Good Laboratory Practice”.
The CA620-CellGuard is intended to be used especially in blood banks as pre do-
nor screening as well as a quality control tool for PLT concentrates in a blood
bank production facility.
During the installation and set-up, the CA620-CellGuard can be configured either
for pre-donor screening, in which the instrument is fully identical to the CA620,
or for quality control to measure PLT concentrates.
1.2 Counter Indications
• Do not use the instrument outdoors.
Usage outside the specified temperature and humidity range might result in
instrument malfunction or short circuit.
- Turn off the power immediately and contact your service department.
• Do not modify the instrument.
Modification without written instructions from the manufacturer might
cause erroneous results or risk for electrical shock.
• Do not remove the cover. Only authorized service personnel is allowed to
open the instrument.
• Do not use the instrument for other purposes then as indicated in this man-
ual.
1.3 Warranty Limitations
• Service and instrument maintenance must be performed by Boule Medical
AB or authorized service personnel. (Referred to hereafter in this manual as
Boule).
• Use only original spare parts and by Boule authorized reagents, blood, cali-
brators and cleaners.
Boule has designed the Medonic instruments as systems for optimal per-
formance. Substituting reagents, calibrators, controls, and components not
recommended by Boule may adversely affect the performance of the instru-
ment. If the substituted products are defective or adversely affect the per-
formance of the instrument it may void your warranty. Each Boule system
is tested at the factory using our recommended reagents, calibrators and
controls, all performance claims are generated as part of this complete sys-
tem.
• System operators and laboratory supervisors are responsible that Boule
products are operated and maintained in accordance to the procedures de-
scribed in the Product Labelling (manuals, package inserts and bulletins of
any kind). They are also responsible for determining that product perform-
ance conforms to the applicable claims.
1070en01

Safety Instruction
10 03-11-24 1070en01
If, under these prescribed conditions of operation and maintenance, an aberrant
or abnormal result occurs, as defined by the laboratory protocol, laboratory per-
sonnel should first make certain that the system is performing and is being oper-
ated in accordance with the Product Labelling. The laboratory protocol should
than be followed to advise the clinician in case a result appears to have deviated
from the norms established by the laboratory.
Boule products do not make diagnosis on patients. Boule intends its diagnostic
products (systems, software and hardware) to be used to collect data reflecting
the patient's hematological status at a certain point of time. Such data may be used
in conjunction with other diagnostic information and with the attending physi-
cians evaluation of the patients condition to arrive at a diagnosis and a clinical
course of treatment.
1.4 General Warnings
Boule incorporates safety features within the instrument in order to protect the
operator from injury, the instrument from damage and the test results from inac-
curacies.
Electrical hazard
• Do not spill blood, reagent, drop metal objects such as wire staples or paper
clips into the instrument. This might cause a short circuit.
If this should occur, turn off the power immediately and contact your serv-
ice department.
Note: The cover should only be opened/removed by authorised service
personnel.
• Do not touch the electrical circuits inside the cover.
There is a hazard of electrical shock.
Note: The cover should only be opened/removed by authorised service
personnel.
Potentially biohazardous material
Because no test method can offer complete assurance that HIV, Hepatitis B or C
viruses, or other infectious agents are absent, these products should be handled
at the Biosafety Level 2 as recommended for any infectious human blood speci-
mens in Protection of Laboratory Workers From Infectious Disease Transmitted
by Blood, Body Fluids and Tissues- 2nd Edition, Tentative Guidelines(1991)
Document M29-T2 promulgated by the National Committee for Clinical Lab.
Standards in the U.S.A. (NCCLS)
Contamination hazard
• Always wear protective gloves and/or goggles when handling infectious or
potentially infectious materials.
• Handle samples with great care.
There is a risk of infection if contaminated blood splashes.
- If blood splashes, enter your eye or a cut, wash it off with plenty of water.
• Do not touch the waste liquid when discarding waste or disassembling/as-
sembling the related parts outside the instrument.
Risk of infection from contaminated blood.
-If you should touch the waste liquid inadvertently, wash off with disinfect-
ant first, then wash it off with soap.
Safety Instruction
1070en01 03-11-24 11
• When handling reagents.
- If a reagent happens to enter your eye, wash it off immediately using plenty
of water and take action to seek medical treatment at once.
- If it happens to adhere to the hand or skin of other body parts, wash it off
using plenty of water.
- If you should swallow it inadvertently, take action to seek medical treat-
ment at once.
Crush hazard
• Always switch off the instrument when opening the front “door”. crush
hazard can occur if the option “Cap Piercing Device” is installed in the
equipment. See Signs on Equipment on page 12.
Note: The cover should only be opened/removed by authorised personnel
during operation.
1.5 Emergency Procedure
• In case of emergency due to an obvious malfunction of the instrument e.g.
smoke or liquid coming out from the inside, proceed as follows:
1. Switch off the instrument immediately by:
Pulling out the mains cord from the mains supply.
2. Contact your authorized distributor’s service department immediately.
1.6 Warning Signs in Manual
These warning signs in the manual are used to identify possible hazards and to
call the operators attention to the existence of this condition.
Indicates operating procedures, practices and so
on, that could result in personal injury or loss of
life if not correctly followed.
Indicates operating procedures, practices and so
on that could result in damage or destruction of
equipment if not strictly observed.
Emphasizes operating procedures, practices and
so on that must be followed to avoid erroneous
results.
Warning
Caution
Important

Safety Instruction
12 03-11-24 1070en01
1.7 Signs on Equipment
1. This label indicates that the safety instructions in the manual must be read
before opening the front door of the instrument.
2. This label indicates a potential bio hazard due to blood exposure or possible
contaminated waste.
3. This label indicates a potential crush hazard. Disconnect the instrument
from the mains outlet before opening the front door. Only authorized per-
sonell are allowed to open front door.
4. Serial Number, Voltage/Fuse specifications and CE / UL markings.
4
Front/side view of the Instrument
Open door view of the Instrument
1
Back view of the Instrument
1013.jpg
1022.jpg
1040.jpg
2
2
3

03-11-24 13
2 Specifications
2.1 General
The operator works with a menu from which the desired program is chosen, e.g.
discriminator settings, calibration, cleaning etc.
Two external reagent reservoirs are used:
• Isotonic diluent.
• Hemolyzing reagent.
Whenever one of these external reservoirs is empty, this is indicated on the dis-
play.
The instrument also performs a 3-part WBC differential by means of a cyanide-
free hemolyzing reagent.
A sample memory is available, protected against mains power failures.
Sample search, selective printing and QC options are available.
The instrument is a fully automated hematology analyser designed to measure up
to 20 parameters using whole blood from an open inlet, closed tubes, micro pi-
pettes 20µl or pre-diluted blood.
2.2 Description of Available Parameters
• The number of Red Blood Cells (RBC)
• The number of White Blood Cells (WBC)
• The number of Platelets (PLT)
• The Mean Cell Volume of Red Cells (MCV)
• The Mean Platelet Volume (MPV)
• The Hemoglobin Concentration (HGB)
The size distributions of PLT, RBC and WBC are measured at the same time as
the above-mentioned parameters.
• Hematocrit (HCT)
• The Mean Cell Hemoglobin (MCH)
• The Mean Cell Hemoglobin Concentration (MCHC)
• The Red Cell Distribution Width (RDW%)
• The Lymphocyte concentration in absolute number and per-
centage
(LYMF)
• The MID-sized Cells (e.g.Monocytes) in absolute number and
percentage
(MID)
• The Granulocytes concentration in absolute number and per-
centage
(GRAN)
• The Red Cell Distribution Width (Absolute) (RDWa)
1001en01

Specifications
14 03-11-24 1001en01
The instrument employs the electronic impedance principle for cell counting and
sizing, and a colorimetric method for measuring hemoglobin. A microprocessor
is used to measure the parameters and to size the cells. During the count the pro-
cessor is checking the analysing process for any irregularities. Size distributions
are printed for all populations (RBC, PLT and WBC).
The instrument has as standard a parallel and a serial output, which is user pro-
grammable. From a built-in selectable program the user can choose between dif-
ferent print formats.
A bar-code reader (scanner), available as an option, can be connected to enter the
sample ID automatically.
The instrument is fully automatic, which means that, the system is always pow-
ered on and performs automatically preforms check- and cleaning cycles to min-
imize user maintenance.
Note:
PCT, LPCR, RDWa and PDWa are not established parameters. Their use should
be restricted to research or investigational use only.
2.3 Short-List of Specifications
• The Platelet Distribution Width (Absolute) (PDW)
• The Platelet Macro Range > 12 fl in% (LPCR)
• The Platelet Packed Volume (PCT)
Measuring principle
RBC, WBC, PLT
Impedance
Measuring principle HGB Cyanide free method 540nm
Discriminator Floating programmable
Sampling system Closed shear valve
Parameters reported RBC, MCV, HCT, PLT, MPV HGB, MCH,
MCHC, WBC,
RDW%, LYMF abs, MID abs, GRAN
abs., LYMF%, MID%, GRAN%
RDW abs, PDW abs, LPCR, PCT
Size distributions printed for RBC, PLT and WBC diff.
Aspirated blood volume
(open tubes)
approx. 125µl
Aspirated blood volume
(closed tubes)1approx. 200 µl
Blood volume using the
Micro Pipette Adapter
20 µl
Pre-diluted mode 1:200 to 1:250 using min. 20µl blood
e.g. 20 µl to 5 µl diluent
30 µl to 6 µl diluent
40 µl to 8 µl diluent
CA530 Screen 2x40 character LCD
CA620 Screen Graphic LCD display
Keyboard Numerical
Total cycle time approx. 73 seconds
Important
Applicable for CA620-
CellGuard:
The MPA is intended to be
used for pre-donor screen-
ing. Using the MPA for
PLT concentrate may re-
duce the accuracy of the
result.

Specifications
1001en01 03-11-24 15
Sample display and print after approx. 53 seconds
Printer External, IBM proprinter, Epson format,
HP-PCL or DPU411-type II /DPU414
supplied by Boule-Medical
CA530 Memory >350 samples
CA620 Memory >200 samples and 600 control samples
CA530 QC capability SD, CV, Xm
CA620 QC capability SD, CV, Xm, Levey-Jennings plots and
X-B with approx. 10.000 samples history
HGB correction on high
WBC counts
Yes
Warning flags on parameter abnor-
malities
Yes
Floating discriminator RBC/ PLT Yes (position printed)
Mathematical 3-part diff. WBC calc. Yes
Automatic HGB blank on each
sample
Yes
Carry Over < 1%
Bar-code reader input Yes
Serial output2Yes
Mains voltage / Fuses 230V Fuse 5x20mm T2A
120V Fuse 5x20mm T4A
Mains voltage tolerances +15% / -15%
Power consumption max 250 VA
Power consumption (stand-by) max 50 VA
Frequency 50/60 Hz
Built-in test /adjustment programs Yes
Temperature 18-32C, 64 - 90F
Humidity (none condensing) 20-80%
Dimensions H= 350,W=420, D=460 (mm),
H= 13.8, W=16.5, D=18.1 (inch)
Weight (net) approx. 22 Kg, 48.5 Lbs
1. Optional device
2. Computer & connection must conform to standard EN 60950

Specifications
16 03-11-24 1001en01
2.4 Parameter Ranges
Linearity +/-1% within the following range:
WBC 0.5 - 80.0
RBC 0.5 - 9.99
MCV 55 - 130
PLT 30 - 999
HGB 0.5 - 55.0
PLT-Concentrate mode
(RBC < 0.3 and measured on a Latex
solution)
30-6999
(applicable on CA620-Cellguard only)
Measuring range:
WBC 0 - 99.9
RBC (CA620-Cellguard) 0 - 13.99
RBC (CA620/530) 0 - 13.99
MCV 15 - 250
PLT 0 - 6999
HGB 0 - 99.9
PLT Concentrate mode 0-6999
(applicable on CA620-Cellguard only)
Correlation1
1. Using Coulter AcT and Abbot CD1600 as reference
RBC, WBC R > 0.97
PLT R > 0.90
HGB R > 0.99
MCV R > 0.90
Reproducibility (typical):
Measured as an average of 10 measurements each of 10 different vein K2-
EDTA collected normal samples:
Parameter X-mean (CGS units) CV(%)
WBC 7.4 2.0
RBC 4.52 0.85
MCV 89.4 0.5
PLT 252 3.2
HGB 13.9 0.8
Specifications
1001en01 03-11-24 17
2.5 Reagents and Reagent Consumption
The reagent consumption is depending on the total number of samples/day. Us-
ing the system for more than ca. 50 samples/day; the reagent consumption will
be:
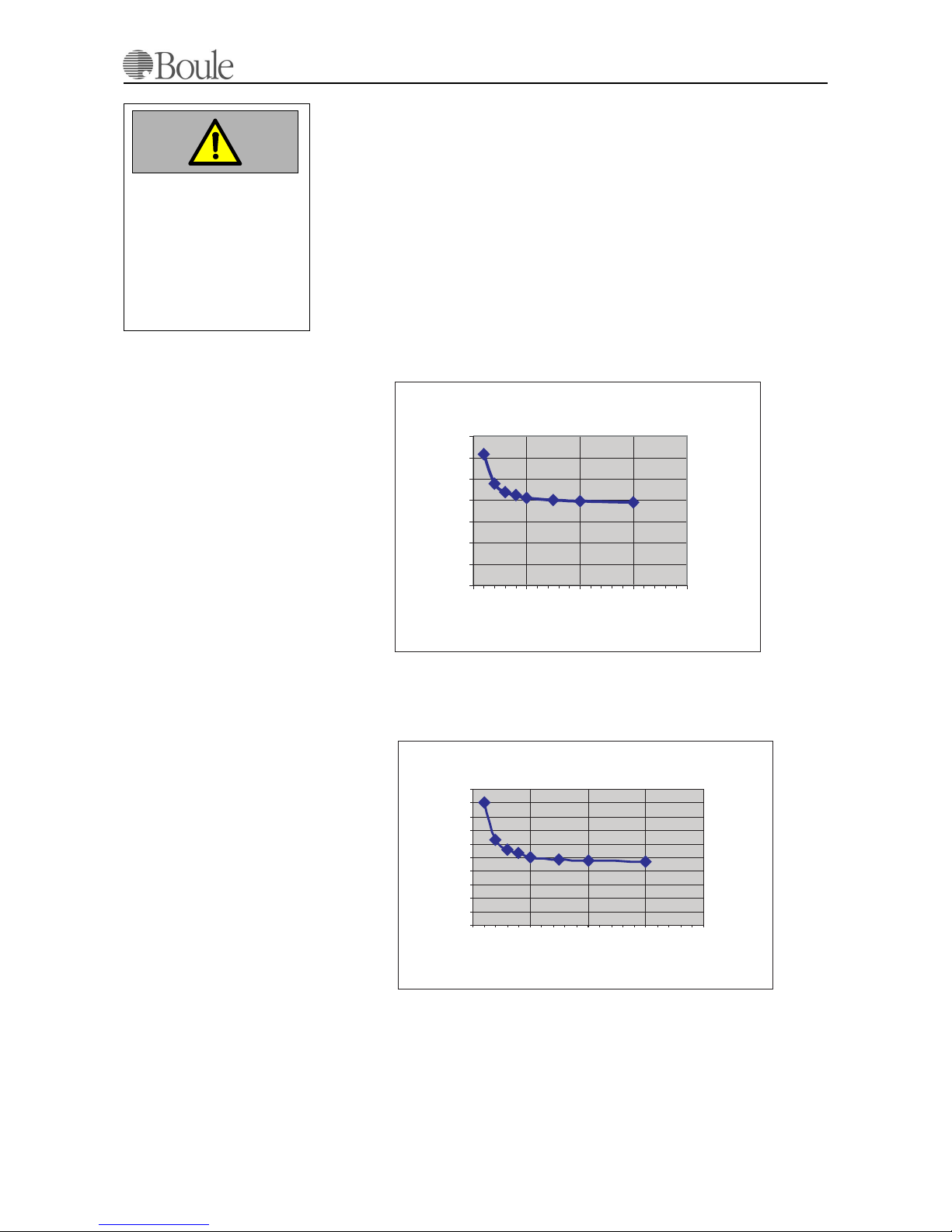
The approximate consumption can be calculated from the graph below and is de-
pending on the number of samples/day. The figures stated are indicative only and
assumes maximum 2 “blank” counts per day. As seen from the graph, the relation
Diluent/Lyzer is 2:1 in case of 50 samples/day or more. Hence, using the instru-
ment for less number of samples/day, less Diluent is consumed in relation to the
lyzer
Diluent Consumption
Lyzer consumption
For additional information regarding cleaning solutions; please refer to Mainte-
nance, Shut-Down & Transport on page 95.
Diluent approx. 19 ml/sample
Lyzer approx. 9.5 ml/sample
Caution
Use only by Boule autho-
rized reagents.
Erroneous results and
damage of the system may
occur if other reagents are
used.
Diluent consumption CA620 vs # samples
0
5
10
15
20
25
30
35
0 50 100 150 200
Number of Blood_samples/Day
Diluent consumption / sample
(ml)
1014.eps
Lyzer consumption CA620 vs # Samples
0
2
4
6
8
10
12
14
16
18
20
0 50 100 150 200
Number of Blood_samples/Day
Lyzer consumption /
sample (ml)
1015.eps

Specifications
18 03-11-24 1001en01
2.6 Limitations
Verification of any abnormal test result (including flagged results or results out-
side of the normal range) should be performed using reference methods or other
standard laboratory procedures for the conclusive verification of results. The sec-
tion below list known limitations of automated blood cell counters in general as
well as specific issues for cell counters using the impedance technology.
WBC White Blood Cells (Leukocytes)
WBC that exceeds the linearity limits of the system will require dilution of the
blood sample. Re-assaying the diluted sample will help to obtain the correct assay
value.
NRBC
Immature Nucleated Red Blood Cells will be counted in the WBC parameter. If
the number of the NRBC is sufficient to activate the DE alarm, such interference
will be detected. However, the manual differential blood cell count, performed
on a stained blood film, will reveal the presence of NRBCs.
Unlyzed Red Cells
In particularly rare instances, the RBC in the blood sample may not completely
lyze. These non-lyzed cells may be detected on the WBC histogram with a DE
alarm or as an elevated baseline on the side of the lymphocyte population. Non-
lyzed RBCs will cause a falsely elevated WBC count. (See also NRBC above)
Multiple myeloma
The precipitation of proteins in multiple myeloma patients may give elevated
WBC counts.
Hemolyzis
Hemolyzed specimen contains red cell stroma (debris), which may elevate the
WBC and/or PLT count.
Leukemias
This disease state may result in a spurious low WBC count because of the pos-
sible increased fragility of the leukocytes leading to some destruction of these
cells during counting. The cell fragments will also interfere with the white cell par-
tial differential parameters (LYMF, GRAN and MID). A spurious low WBC
count may also be seen in patients with lymphocytic leukemias due to the pres-
ence of abnormally small lymphocytes, which may not be counted by the instru-
ment.
Chemotherapy
Cytotoxic and immunosuppressive drugs may increase the fragility of the leuko-
cytes, which may cause low WBC counts.
Cryoglobulins
Increased levels of cryoglobin that may be associated with myeloma, carcinoma,
leukemias, macroglobulinemia, lymphoproliferative disorders, metastatic tu-
mours, auto immune disorders, infections, idiopathic disease, aneurism, pregnan-
cy, thromboembolic phenomena, diabetes etc. and may cause elevated levels of
WBC, RBC or PLT counts as well as HGB. The specimen can be warmed up to
37°C and re-analysed immediately or a manual WBC, RBC or PLT count can be
performed.

Specifications
1001en01 03-11-24 19
RBC Red Blood Cells (Erythrocytes)
The red blood cell dilution contains all the cellular elements of the blood, RBC,
WBC and PLT. During the counting platelets, are not counted since the size falls
below the threshold (discriminator). Leukocytes however, are always included in
the RBC count. Since the concentration ratio between RBC and WBC is normally
ca. 1000, the introduced error is almost negligible.
In case of high WBC counts with low RBC, the RBC count may be corrected by
simply subtracting the WBC from RBC.
Agglutinated red blood cells
This might cause a falsely decreased RBC count. Blood samples containing the
agglutinated red blood cells may be identified by observing abnormal MCH and
MCHC values as well as by examination of the stained blood film.
Cold agglutinins
IgM immunoglobulins which are elevated in cold agglutinin disease may lower
RBC and PLT counts and increase the MCV.
HGB (hemoglobin)
Turbidity of the blood sample due to any number of physiological and/or thera-
peutic factors may produce falsely elevated HGB results. The CA620/530 how-
ever is compensated for this effect with high WBC counts up to WBC approx. 60
10e3/µl. In case of extreme WBC counts, the following is recommended:
1. Elevated WBC
The diluted sample should be centrifuged and the supernatant fluid checked
on a spectrophotometer for turbidity.
2. Elevated lipids
Elevated lipids in the blood sample will give the plasma a “milky” appear-
ance. This condition can occur with hyperlipidemia, hyperproteinemia and
hypobilirubinemia. Accurate HGB determination can be achieved by using
reference methods and a plasma blank.
Increased turbidity may also be seen in cases where the red blood cells are resis-
tant to lyzing. This condition will cause a falsely elevated HGB result but can be
detected by monitoring the MCHC.
Fetal blood
The mixing of fetal and maternal bloods may produce a falsely elevated HGB val-
ue.
HCT (Hematocrit)
As HCT is the product of MCV x RBC, any erroneous result in MCV and/or
RBC will produce an equal error in the HCT parameter.
Red blood cell agglutination
May produce an erroneous MCV value and therefore a false HCT
WBC
An excessive number of WBCs might cause interference within the RBC popula-
tion and therefore a false MCV value.
PLT
Excessive numbers of PLT, in most cases, do not interfere with the MCV param-
eter due to the use of the floating discriminator technology in the instrument.

Specifications
20 03-11-24 1001en01
RDW (Red Cell Distribution Width)
The red cell distribution width is a function of the RBC count and derived from
the RBC histogram. In most cases, any error introduced in the MCV may also
cause the RDW to be erroneous. Especially blood transfusions may raise the
RDW significantly due to the presence of bi-modal populations.
PLT (Platelets or Thrombocytes)
Very small RBCs might elevate a PLT count. This effect is minimized in the in-
strument due to the use of a floating threshold (discriminator). By observing the
PLT and RBC histograms, this effect is seen as an overlapping PLT/RBC area.
Agglutinated RBCs
Agglutinated RBCs might trap platelets and may give an erroneous low PLT
count. The presence of agglutinated RBCs is detected by monitoring the MCHC
parameter and by careful examination of the stained blood film.
Giant platelets in excessive numbers
This may cause a low PLT count since they might fall within the RBC threshold
range.
Chemotherapy
Cytotoxic and immunosuppressive drugs may increase the fragility of these cells,
which may cause low PLT counts. Reference (manual) methods may be necessary
to obtain an accurate platelet count.
Hemolyzis
Hemolyzed specimens contain red cell stroma, which may elevate platelet counts.
A.C.D. blood
Blood anti coagulated with Acid Citrate Dextrose may contain platelet aggre-
gates, which could depress the platelet count.
RBC inclusions
Erythrocyte inclusions may also produce a spuriously increased platelet count.
(e.g. Howell-Jolly bodies, siderotic and basophilic granules)
Platelet agglutination
Clumped platelets due to poor collection techniques or platelet satellitosis caused
by EDTA activation of immunoglobulins may cause a decreased platelet count
and/or an elevated WBC count. The specimen should be recollected in sodium
citrate anticoagulant and re analyzed for only the platelet count. The final PLT
result must be corrected for the sodium citrate dilution effect.
This manual suits for next models
1
Table of contents