2
POTENTIAL COMPLICATIONS
WARNINGS
• Bleeding, perforation, trans-mural burn, thermal injury, respiratory
depression or apnea, arrhythmia or cardiac arrest;
• Infection, septicaemia, etc.;
• Complications which may exist and are not known or observed.
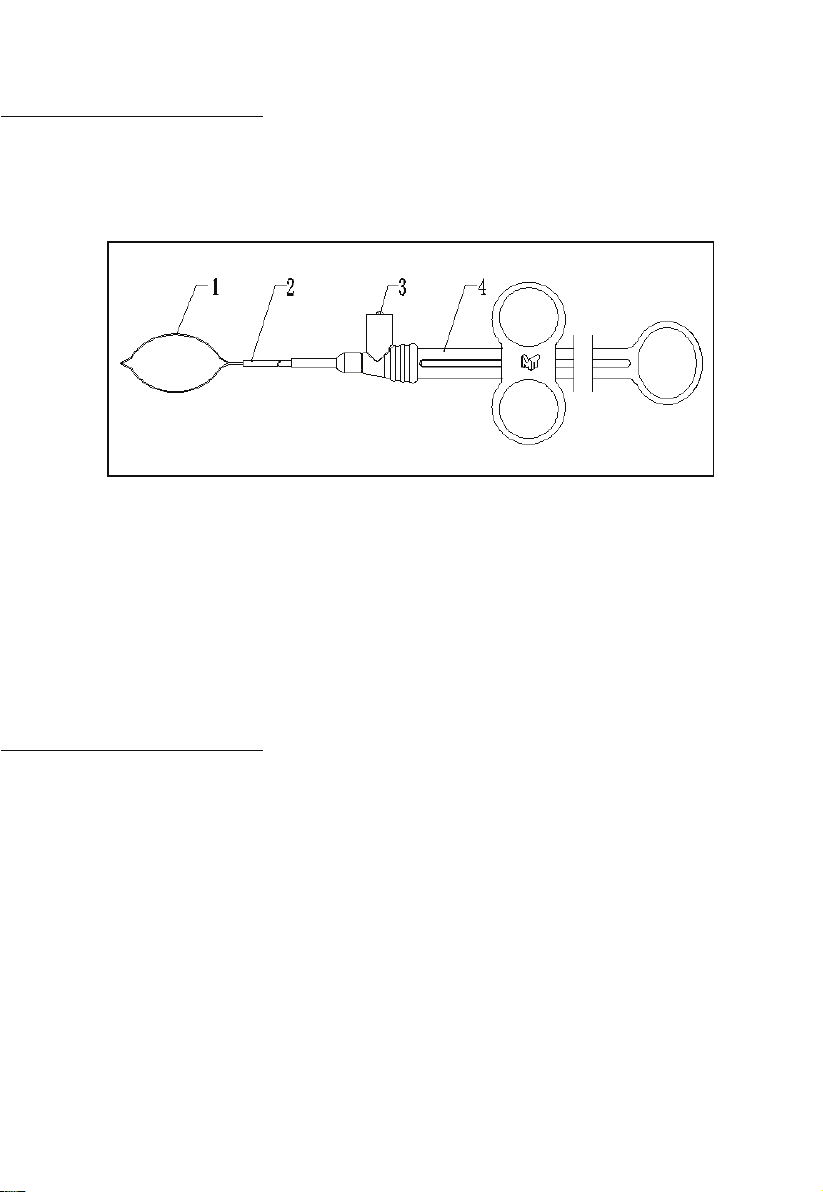
Hot Snare Use:
1. Retract the handle of the Snare and confirm that the Snare loop is fully
retracted into the catheter prior to inserting the catheter into the
endoscope.
2. Advance the catheter through the endoscope channel until the lesion
is clearly identified and targeted. Snare loop could be rotated if
rotatable function is available.
3. With electrosurgical unit off,securely connect the active cord to the
Snare handle and electrosurgical.
4. Turn the electrosurgical unit ON.
NOTE:the device should be installed and put into service according
to the EMC information provided in the electrosurgical unit
accompanying documents.
NOTE:In strict accordance with the applicable electrosurgical device
operational mode and Input feature during operation.
Take the CONMED Electrosurgery 60-8200-230 as an example:
Operational mode: intermittence 15s open/30s close;
The rated Input :100~240V,4A max.50/60Hz.
5. Operate the handle to extend the Snare loop and snare the target
tissue gently. Press the foot switch to active output. Pull the slider and
resect the target tissue. Do not apply excessive force when snaring the
tissue. This could cause patient injury, such as punctures, hemorrhages
1. The product is intended for single use only! DO NOT re-use, re-sterilize,
and/or reprocess. Re-use, re-sterilization or reprocessing may
compromise the structural integrity of the device and/or lead to
device failure which, in turn, may result in patient injury, illness or death.
Re-use, re-sterilization or reprocessing may also create a risk of
contamination of the device and/or cause patient infectious
disease(s). Contamination of the device may lead to injury, illness or
death of the patient. Micro-Tech assumes no liability with respect to
instruments reused, re-sterilized or reprocessed .
2. Do not use this instrument for any purpose other than its intended use.
3. The product is only intended for adult populations.
4. This device is not made with natural rubber latex.
5. Patients should be informed of the potential risks and complications,
which may lead to injury, illness or death of the patient.
6. The instrument is intended for use under the direct supervision of a
suitably trained physician only. A thorough understanding of the
technical principles, clinical applications, and associated risks is
expected before usage.。
7. Confirm that the endoscopy view is clear before use. Do not insert the
instrument into the endoscope unless you have a clear endoscopic
Contraindications include, but may not be limited to:
1. Poor physical fitness, with serious heart or/and lung disease, and can’t
tolerate endoscopy and endoscopic treatment;
2. Bleeding tendency, an extended bleeding and prolonged
coagulation time, or thrombocytopenia or lack of prothrombin which
couldn’t be treated
3. A polyp / polyps with a large base more than 2cm for gastric poly and
1.5cm for normal polyp.
4. A polypoid cancer which has infiltrated into tissue or organ and has
deteriorated;
5. Patients with implanted pacemaker or implanted with a metal valve /
values(It is classified as relative contraindication);
6. Diabetes, regardless of whether blood sugar is normal(poor healing
capacity) (It is classified as relative contraindication);
7. Uncooperative patients or family members.
field of view. Insertion without clear endoscopic field of view could
cause patient injury, such as perforation, hemorrhage or mucous
membrane damage. Damage to the endoscope and/or the
instrument may also occur.
8. When applied to a patient with a pacemaker implanted, the instrument
may cause malfunction or failure of the pacemaker, seriously affecting
the patient. Consulting cardiologist or the manufacturer of the
pacemaker is recommended before instrument application.
9. When using the instrument in the vicinity of the heart, be sure to use it
with the minimum necessary output. Spark discharge during operation
may affect the heart.
10. When using an electrocardiograph or other physiological monitoring
equipment simultaneously with the instrument, any monitoring
electrodes should be placed as far away as possible from the
electrodes used with the electrosurgical unit. Needle monitoring
electrodes should not be used. Physiological monitoring equipment
incorporating high-frequency current limiting device is
recommended.
11. When the endoscope is used with the device, the leakage currents
may be relatively increased. Ensure a proper path from patient return
electrode to electrosurgical unit is connected and maintained
throughout the procedure.
12. Before use, please check the insertion patients body parts, ensure that
no sharp edge.
13. Do not touch energized handle components (e.g. the high-frequency
applied mandrel)and do not contact with conductive object as
shown in Fig.1 and Fig.2 below.
or mucous membrane damage or thermal injury.
6. Retract the snare prior to removing the instrument from the endoscope
7. Upon completion of the procedure, disconnect the active cord from
the Snare handle and dispose of the device according to the
institutional guidelines for biohazard medical waste and local
regulatory requirements.