Miha bodytec m.ove User manual

miha-bodytec m.ove
Operating instructions US

Read this manual prior to performing any task!
© miha bodytec Inc. 2021
miha bodytec Inc.
2171 Executive Drive Suite 200
Addison, Illinois 60101
USA
Telephone: +1 833 367 6442
E-mail: [email protected]
Internet: www.miha-bodytec.us
This manual was created by:
kothes GmbH
Internet: www.kothes.com
TD150168 – Rev. 0
2021-07-28miha-bodytec m.ove EMS training device2

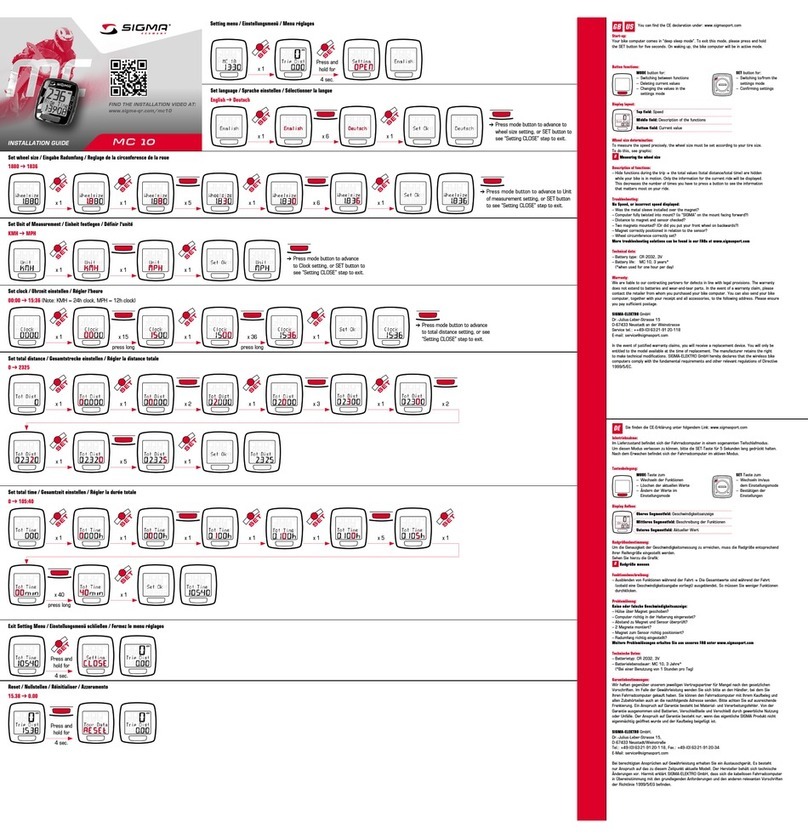
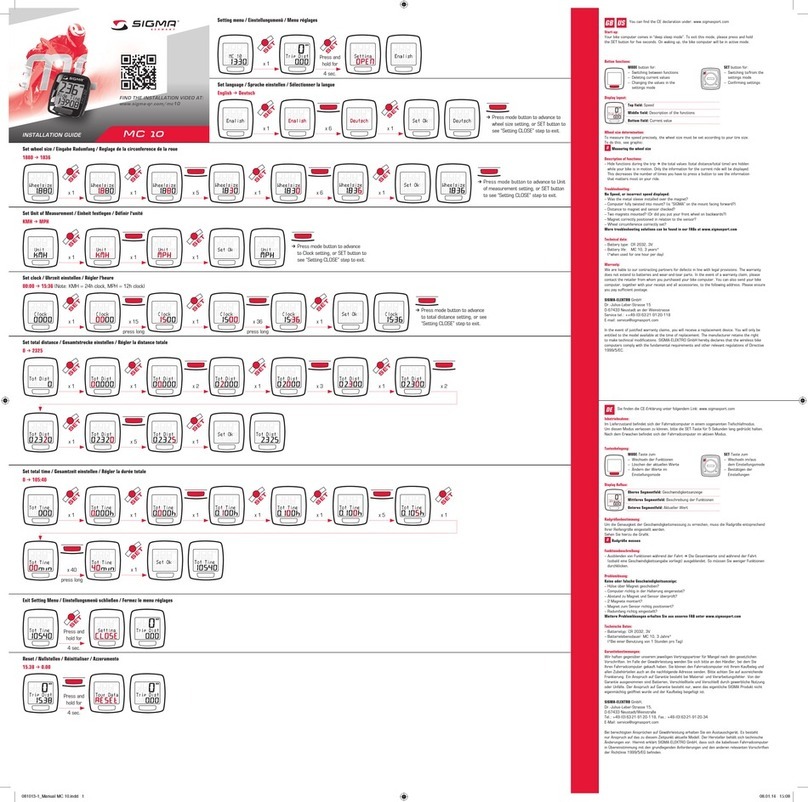
Conscientious handling of the miha bodytec m.ove
Conscientious handling is the prerequisite for successful and safe
use. The trainer must take part in a training session held by the
manufacturer to be able to safely operate the EMS training device,
hereinafter referred to as “device”.
Before starting with the workout, the trainer instructs the athlete/
patient in the basic operating functions and displays on this device
for the workout. The trainer points out consequences caused by
misuse.
Information about this manual
This manual enables safe and efficient handling of the device. This
manual is an integral part of the device and must be kept in the
immediate vicinity of the device and accessible to the trainer at any
time.
The trainer must be able to read and understand this manual. The
trainer must have carefully read and understood this manual before
handling the device. The prerequisite for safe training is compli-
ance with all safety instructions and procedural instructions speci-
fied in this manual. The local regulations for the prevention of acci-
dents and the general safety regulations for the location in which
the device is used also apply.
The illustrations in this manual provide a basic understanding and
may vary from the actual design.
Safety instructions
Safety instructions in this manual are identified by symbols. The
safety instructions are introduced by signal words which express
the extent of the hazard.
DANGER indicates a hazardous situation which, if not
avoided, will result in death or serious injury.
WARNING indicates a hazardous situation which, if not
avoided, could result in death or serious injury.
CAUTION indicates a hazardous situation which, if not
avoided, could result in minor or moderate injury.
NOTICE indicates important, non-safety-related information,
such as property and environmental damage.
Safety instructions in procedural instructions
Safety instructions can refer to specific, individual procedural
instructions. Such safety instructions are embedded in the proce-
dural instructions so that they do not interrupt the reading flow
when performing the activity. The signal words described above
are used.
L DANGER
L WARNING
L CAUTION
NOTICE
Supplemental directives
2021-07-28 miha-bodytec m.ove EMS training device 3

Example:
L WARNING! Risk of injury if detaching the electrodes
while the training program is running!
Make sure that no training program is active. To do so, switch
to the main menu.
Tips and recommendations
This symbol highlights useful tips and recommendations, as well as
useful information for efficient and fault-free operation.
Identifiers in this manual
To highlight procedural instructions, results, lists, references, and
other elements, the following identifiers are used in this manual:
Identifier Explanation
Step-by-step procedural instructions
ðResults of procedural steps
References to sections of this manual and
to other applicable documents
Lists without a defined sequence
[Button] Controls (e.g., buttons, switches), display
elements (e.g., signal lamps)
“Display” Screen elements (e.g., buttons, assignment
of function keys)
The following document applies in addition to this manual:
nUser manual for i-body® connect wireless
nInstallation manual for travel station m.ove
nInstallation manual for work station m.ove
The contents of this manual are protected by copyright. Use of
these contents is permissible within the framework of use of the
device. Any other use is not permitted without the written permis-
sion of the manufacturer.
Other applicable documents
Copyright
Supplemental directives
2021-07-28miha-bodytec m.ove EMS training device4

Table of contents
1 Overview and scope of delivery........................................ 7
1.1 Scope of delivery.......................................................... 7
1.2 Equipment................................................................... 10
2 Safety................................................................................. 17
2.1 Intended use............................................................... 17
2.2 Misuse........................................................................ 18
2.3 Risks due to physical condition: Contraindications..... 19
2.4 A team for your safety................................................. 20
2.5 Warnings..................................................................... 21
2.6 Reporting of adverse events....................................... 29
2.7 Symbols on the miha bodytec m.ove.......................... 30
2.7.1 Symbols on device................................................... 30
2.7.2 Rating plates............................................................ 31
2.7.3 Cleaning symbols.................................................... 34
2.8 Environmental protection............................................ 35
3 Technical data................................................................... 36
4 Electromagnetic compatibility......................................... 42
4.1 Compliance details..................................................... 42
4.2 EMC Warnings............................................................ 46
5 Basic information on EMS training................................. 48
5.1 Safety first................................................................... 48
5.2 EMS training............................................................... 48
5.3 Training frequency and regeneration.......................... 49
6 Understanding the device................................................ 52
6.1 Explanation of terms................................................... 52
6.2 Opening menu tabs.................................................... 55
6.3 Opening menus.......................................................... 56
6.4 Entering text................................................................ 59
7 Customization and setup................................................. 61
7.1 Adjusting the device setup.......................................... 61
7.2 Selecting the language............................................... 62
7.3 Program memory settings........................................... 63
7.4 Setting up the training plan memory........................... 63
7.5 Setting up synchronized start..................................... 64
7.6 Adjusting the favorites menu...................................... 66
7.7 Backing up data.......................................................... 67
7.8 Restoring/importing data............................................. 68
7.9 Installing updates........................................................ 69
7.10 Using networks......................................................... 70
7.11 Resetting to factory settings...................................... 71
7.12 Displaying statistics.................................................. 71
Table of contents
2021-07-28 miha-bodytec m.ove EMS training device 5

8 Before the training............................................................ 72
8.1 Eating and drinking..................................................... 72
8.2 Selecting undergarments and shoes.......................... 72
8.3 Moistening and applying electrodes........................... 73
9 Training with the device................................................... 83
9.1 Safety during training.................................................. 83
9.2 Connecting the device................................................ 84
9.3 Operation with the rechargeable battery..................... 85
9.4 Individual training settings.......................................... 85
10 Overview of programs/training plans............................. 87
10.1 Selecting individual training settings......................... 87
10.2 Stress standards....................................................... 87
10.3 Training programs..................................................... 88
10.4 Training plans........................................................... 89
10.5 Training..................................................................... 94
10.6 Transponder system................................................. 98
10.7 Training with synchronized start............................. 102
11 After the training............................................................. 103
11.1 Taking off electrodes............................................... 103
11.2 Cleaning and storage.............................................. 104
11.2.1 Cleaning the i-body® and the individual elec-
trodes................................................................... 104
11.2.2 Cleaning the external cables................................ 106
11.2.3 Cleaning the control unit...................................... 107
12 Packaging and storage................................................... 108
12.1 Symbols on the packaging...................................... 108
12.2 Transport and storage............................................. 109
12.3 Environmental protection........................................ 110
13 Ensuring optimum function........................................... 111
13.1 Maintaining the device............................................. 111
13.2 Maintaining the electrodes...................................... 112
13.3 Handling error messages........................................ 112
14 Index................................................................................. 113
Table of contents
2021-07-28miha-bodytec m.ove EMS training device6

1 Overview and scope of delivery
1.1 Scope of delivery
2
1
3
4
5
Fig. 1: Overview of miha bodytec m.ove
1Control unit (
Ä
“Control unit (top side)”
on page 8)
2 Power supply
3 Power cable
4 On/off switch
5 Connection for power supply unit incl. cover
EMS stands for ElectroMyoStimulation. This means that the mus-
cles are stimulated by electrical pulses. Complete body training
which addresses all muscle groups is possible with up to 10 pairs
of electrodes.
During conventional training, electrical signals from the brain ini-
tiate contractions and thus movement of the muscles. With EMS,
electrical pulses from outside activate the muscles. It makes no dif-
ference to the muscle whether electrical stimuli are sent from the
brain or from electrodes: It reacts by contracting.
miha bodytec m.ove
What is EMS training?
Overview and scope of delivery
2021-07-28 miha-bodytec m.ove EMS training device 7

Special features and benefits:
nAll muscle groups can be activated using an electrode system
with up to 10 pairs of electrodes.
nStatic and dynamic training with interaction of deliberate muscle
contraction and EMS.
nExercise postures increase the contraction of the deliberately
stimulated muscles.
nPositive and negative electrodes are not on the same muscle.
nAgonist and antagonist are stimulated simultaneously.
nEMS training can cause more intense muscle contractions than
classic strength training. At the same time, there is compara-
tively very little stress on the joints.
nDeeper muscle groups can be easily reached.
nThe training is very intense and thus short (10 – 20 minutes).
Fig. 2: Control unit (top side)
1 Level controller for legs
2 Level controller for buttocks
3 Level controller for lower back
4 Level controller for upper back
5 Level controller for side back
6 Level controller for abdomen
7 Level controller for chest
8 Level controller for arms
9 No function assigned
10 No function assigned
11 LC display
12 Transponder card contact surface
13 Multi-function button for start/stop and for setting the programs
and the program parameters
14 Main controller for setting the pulse strength
Control unit (top side)
Overview and scope of delivery
2021-07-28miha-bodytec m.ove EMS training device8

All controls with the exception of the on/off switch, which is located
on the underside (Fig. 3), are located on the top side of the control
unit.
The main controller (Fig. 2/14) enables the athlete to regulate
pulse strength of all outputs simultaneously. A connected pair of
electrodes is an output in this context.
The pulse strength of individual outputs can be regulated using the
relevant level controllers (Fig. 2/1 – 8). The image above each of
these rotary switches shows the relevant part of the body for which
the pulse strength is regulated. For example, using the level con-
troller for legs (Fig. 2/1), the pulse strength sent to the legs is regu-
lated using the i-body® straps flex.
The two level controllers without any assigned body parts (Fig. 2/9,
10) do not have any function.
If a transponder card is placed on the transponder card contact
surface (Fig. 2/12), the settings stored on this card can be edited or
loaded automatically (
Ä
Chapter 10.6 “Transponder system”
on page 98).
The multi-function button (Fig. 2/13) is used for navigating through
the menus and for activating programs. The multi-function button is
also used for starting and stopping workouts.
The LC display (Fig. 2/11) shows menus and provides status infor-
mation on the device. A virtual person – an avatar – is shown on
the LC display during training. The athlete can use the movements
of the avatar, in addition to the instructions of the trainer, as an ori-
entation.
Overview and scope of delivery
2021-07-28 miha-bodytec m.ove EMS training device 9
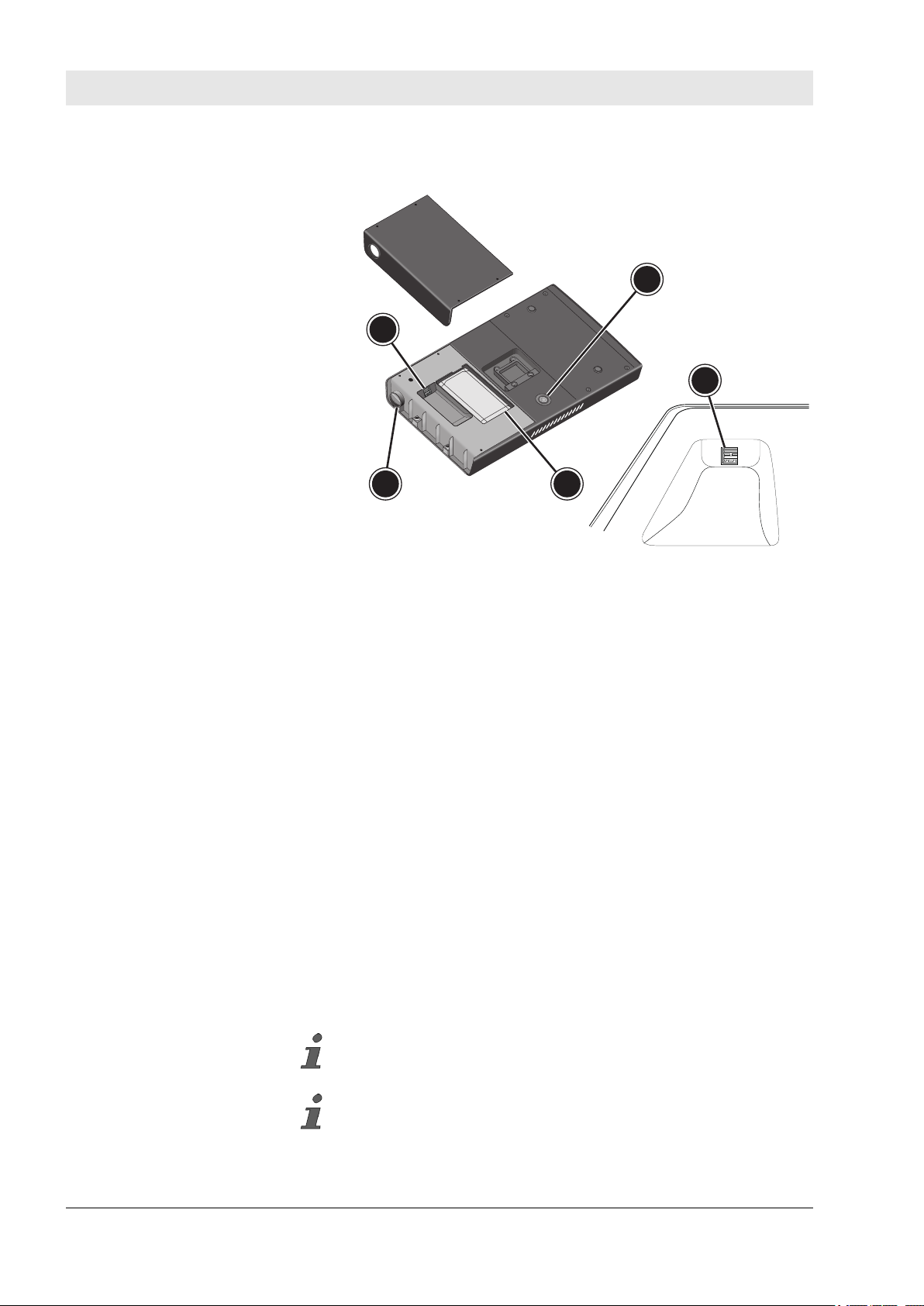
3
3
2 4
1
Fig. 3: Control unit (underside)
1 On/off switch
2 Power supply connection incl. cover
3 USB port
4 Rechargeable battery
The on/off switch (Fig. 3/1) is embedded in the case on the under-
side of the control unit. A built-in terminal strip is located in a
depression on the underside, which is protected by a service cover.
The supply cable of the power supply unit is connected to the
power supply connection (Fig. 3/2) by means of a magnetic plug
connection.
The miha bodytec USB flash drive can be inserted into the USB
port (Fig. 3/3) for performing updates and for saving device set-
tings.
The rechargeable battery (Fig. 3/4) located in a designated
rechargeable battery compartment incl. connector is used to supply
power without a power supply during mobile use.
1.2 Equipment
Using equipment not obtained from miha bodytec or authorized
dealers represents an increased safety risk. Only use genuine
miha bodytec equipment.
It is not allowed to connect additional parts not mentioned in this
manual to the device, the electrodes or the cables.
Control unit (underside)
Safety thanks to genuine miha
bodytec equipment
Overview and scope of delivery
2021-07-28miha-bodytec m.ove EMS training device10

Rechargeable battery for miha bodytec m.ove
Mobile floor stand for storing the equipment, with a holder for the
control unit when in mobile use.
For further information, please refer to TD 15303 – Installation
instructions for travel station m.ove.
Rechargeable battery for m.ove
Fig. 4: Rechargeable battery for
m.ove
travel station m.ove
Fig. 5: travel station m.ove
Overview and scope of delivery
2021-07-28 miha-bodytec m.ove EMS training device 11

Medical cart for storing the equipment, with a holder for the control
unit when in mobile use in specific facilities.
For further information, please refer to TD 15304 – Installation
instructions for work station m.ove.
Wireless stimulation unit that can be attached to the body of the
athlete for wireless training via a Bluetooth® connection.
See the “i-body® connect wireless” manual for further information.
work station m.ove
Fig. 6: work station m.ove
i-body® connect wireless
Fig. 7: i-body
®
connect wireless
Overview and scope of delivery
2021-07-28miha-bodytec m.ove EMS training device12

Charger for i-body® connect wireless.
See the “i-body® connect wireless” manual for further information.
Transponder card for storing the last values, individual time specifi-
cation, and individually adjusted program.
The i-body® electrode vest for applying electrodes to the upper
body.
Table 1: Electrode sizes for each vest size
Vest size Size 1 and size V1 Size 2, size 3 and
size V2 in in²
2 electrodes for
abdomen 22.85 in² 27.56 in²
2 electrodes for
chest 10.31 in² 12.81 in²
i-body® connect charger
Fig. 8: i-body
®
connect charger
Transponder card
Fig. 9: Transponder card
i-body®
Fig. 10: i-body
®
Overview and scope of delivery
2021-07-28 miha-bodytec m.ove EMS training device 13

Vest size Size 1 and size V1 Size 2, size 3 and
size V2 in in²
2 electrodes for
upper back 16.25 in² 20.98 in²
2 electrodes for
sides of back 9.75 in² 11.85 in²
2 electrodes for
lower back 14.4 in² 19.47 in²
Electrodes for applying to arms and legs.
Table 2: Electrode sizes for each i-body
®
straps flex size:
Size of the i-body®straps flex Electrode size
Size 1 (pair) 16.74 in² each
Size 2 (pair) 20.77 in² each
Size 3 (pair) 29.14 in² each
Buttocks electrode, hereinafter referred to as “i-body® belt”, for
applying to the buttocks.
Table 3: Electrode sizes for each i-body
®
belt size:
Size of the i-body®belt Electrode size
Size 1: 2 buttocks electrodes each 14.7 in²
Size 2: 2 buttocks electrodes each 18.34 in²
i-body® straps flex
Fig. 11: i-body
®
strap flex
i-body® belt
Fig. 12: i-body
®
belt
Overview and scope of delivery
2021-07-28miha-bodytec m.ove EMS training device14

External cables, hereinafter referred to as “i-body® cables” for con-
necting the “i-body®” to the external “i-body® straps flex” and “i-
body® belt” electrodes.
1 Pants
2 Top
Undergarments for wearing under all electrodes to be applied.
Pump spray bottle for moistening the electrodes.
The miha bodytec USB flash drive is delivered with the device and
serves as a data carrier for performing updates as well as saving
and transferring settings.
i-body® cable
Fig. 13: i-body
®
cable
Undergarments
Fig. 14: Undergarments
Pump spray bottle
Fig. 15: Pump spray bottle
miha bodytec USB flash drive
Overview and scope of delivery
2021-07-28 miha-bodytec m.ove EMS training device 15

In the event of important updates, a miha bodytec USB flash drive
will be sent to users of the device who perform the update them-
selves or have it performed on the device by the trainers.
For technical information, please contact us at our headquarters:
Address miha bodytec Inc.
2171 Executive Drive Suite 200
Addison, Illinois 60101
USA
Telephone +1 833 367 6442
E-mail [email protected]
Website www.miha-bodytec.us
We are also always interested in information and experience
arising from the use of the device which can be valuable for the
improvement of our product.
Customer service
Overview and scope of delivery
2021-07-28miha-bodytec m.ove EMS training device16

2 Safety
2.1 Intended use
miha bodytec m.ove is a device which performs electronic muscle
stimulation based on EMS technology. The device is specifically
designed as an addition to other sports and for training muscles.
miha bodytec m.ove is intended to stimulate muscles in order to
improve or facilitate muscle performance. In addition it is indicated
for the following conditions:
nRe-educating muscles
nRelaxation of muscle spasm
nRetarding or preventing disuse muscle atrophy
The miha bodytec m.ove electrical impulses allow the triggering of
action potentials on motoneurons of motor nerves (excitations).
These excitations of motoneurons are transmitted to the muscle
fibers via the motor endplate where they generate mechanical
muscle fiber responses that correspond to muscle work.
Depending on the parameters of the electrical impulses (pulse fre-
quency, duration of contraction, duration of rest, total session dura-
tion), different types of muscle work can be imposed on the stimu-
lated muscles.
miha bodytec m.ove may only be used by persons above the age
of 21.
Safety
2021-07-28 miha-bodytec m.ove EMS training device 17

2.2 Misuse
Danger in the event of misuse!
– Keep the device out of the reach of children.
– Never apply electrodes to positions other than those
described in this manual.
– Never use the electrical stimulation treatment in the fol-
lowing areas of the body:
– On or through the head
– Directly on the eyes
– In areas around the mouth
– On the front of the neck (especially the carotid artery)
– With electrode surfaces applied to the chest and the
upper back or across the heart
– Over the menstruating or pregnant uterus
– Over areas of the skin with a lack of normal sensation.
– Stimulation must not be applied over the carotid sinus
nerves, particularly in patients with a known sensitivity to
the carotid sinus reflex.
– Stimulation must not be applied over the neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles
may occur and the contractions may be strong enough to
close the airway or cause difficulty breathing.
– Stimulation shall not be applied transthoracically. The intro-
duction of electrical current into the heart may cause car-
diac arrhythmias.
– Stimulation must not be applied transcerebrally.
– Stimulation must not be applied over swollen, infected, or
inflamed areas or skin eruptions, e.g., phlebitis, thrombo-
phlebitis, varicose veins, etc.
– Stimulation must not be applied over, or in proximity to,
cancerous lesions.
– Whenever using the device, start with an intensity level of
“0” and increase slowly.
– Ensure basic tension in the muscle group you are exer-
cising for each pulse to prevent uncontrolled muscle con-
tractions.
– In case of skin irritation and signs of burns beneath the
electrodes, stop using the device immediately.
– If you are feeling unwell, dizzy, or have heart pain, stop
using the device immediately.
– Some patients may experience skin irritation or hypersensi-
tivity due to the electrical stimulation or electrical conduc-
tive medium. The irritation can usually be reduced by alter-
nate electrode placement using an alternate conductive
medium.
– Electrode placement and stimulation settings must follow
guidance of the prescribing practitioner.
– The long-term effects of repeated electrical stimulation are
unknown.
– Only use genuine miha bodytec equipment and spare parts.
L WARNING
Safety
2021-07-28miha-bodytec m.ove EMS training device18

Misuse of the device can cause hazardous situations. The
device is exclusively intended for use as an electronic muscle
stimulator for the usecases described in this manual and must
only be used in a dry environment. Skin irritation and burns
beneath the electrodes have been reported with the use of
powered muscle stimulators.
2.3 Risks due to physical condition: Contraindications
Risk of fatal injury in the event of use despite presence of
contraindications for specified persons!
All persons training with the device must ensure that none of
the following contraindications are present:
– Before each further training session the trainer must check
if the health condition of the athletes/patients has changed
and/or if they are under influence of alcohol, drugs, nar-
cotics, and/or painkillers.
– In the event of doubts whether any contraindication is pre-
sent, do not use the device.
– In the event of doubts, consult a doctor before the first or
any further trainings.
– If you are taking medication, consult a doctor before the
first or any further trainings.
The presence of certain physical conditions can result in a
risk of injuries or even death. The existence of such a physical
condition is referred to as contraindication.
The device must not be used if the following contraindications are
present:
nAcute influence of alcohol, drugs, narcotics, and/or painkillers
nRecent operations
nCardiac arrhythmias
nActive medical implants
nEpilepsy
nSeizures
nPregnancy or suspected pregnancy
nSevere circulatory disorders
nArterial circulatory disorders
nStrong bleeding tendencies (hemophilia)
nBleeding
nAbdominal wall hernia
nInguinal hernia
nTuberculosis
nTumor diseases
nArteriosclerosis in advanced stage
nSevere neurological disorders
EMS training in the event of
existing contraindications
L WARNING
Never exercise if ...
Safety
2021-07-28 miha-bodytec m.ove EMS training device 19

nDiabetes mellitus
nFebrile diseases
nAcute bacterial or viral infections
nLiver diseases
nKidney diseases
nCardiovascular diseases
nCoronary heart diseases
nInfected or wounded areas of the skin
nSkin cancer
nRhabdomyolysis
2.4 A team for your safety
This section describes the requirements for people involved in
training with this device. To ensure safe EMS training, the actions
described in this manual may only be performed when the relevant
person meets the specified requirements.
Athlete/Patient
Athletes/patients use the device actively for EMS training. They
make sure that they are instructed by a qualified trainer of the
training-relevant control elements and displays and notified of the
consequences caused by misuse. They also make sure that they
are guided through the training session by the trainer. Athletes/
patients must provide the trainer responsible for them with truthful
information on current health restrictions (e.g., fever).
Athletes/patients make sure that none of the listed contraindica-
tions (
Ä
Chapter 2.3 “Risks due to physical condition: Contraindi-
cations” on page 19) apply to them before they start with the
training.
Trainer
The trainer must have taken part in a basic miha bodytec training
session and can prove their qualification by presenting a certificate
issued in their name. The participation in an additional higher-level
EMS training session is recommended. This following information
is covered during this training session:
nEffects and special characteristics of EMS training
nAdjustable parameters of the device and their impact
nIdentifying and avoiding risks:
– Function-related residual risks
– Settings-related residual risks
– Residual risks due to the condition of the athlete/patient
nAvailable equipment and its correct usage
nApplying the electrodes
nFunction, operation, and programming of the control unit
nPerforming updates
Acting responsibly
Safety
2021-07-28miha-bodytec m.ove EMS training device20
Table of contents