9. support@minipcr.com
bluegelTM user’s guide
RUNNING A GEL
Place the gel tray containing a gel in the buffer chamber and place the buffer chamber
inside the blueGelTM base. The wells should be closest to the (-) end.
Add 30 ml of 1X TBE buffer in the buffer chamber. The buffer should just cover the
agarose gel.
CAUTION: Do not overll the gel chamber as it may overow when the cover is placed
over the gel.
Remove air bubbles (if any) trapped between the gel and the gel tray, or between the
gel tray and the buffer chamber.
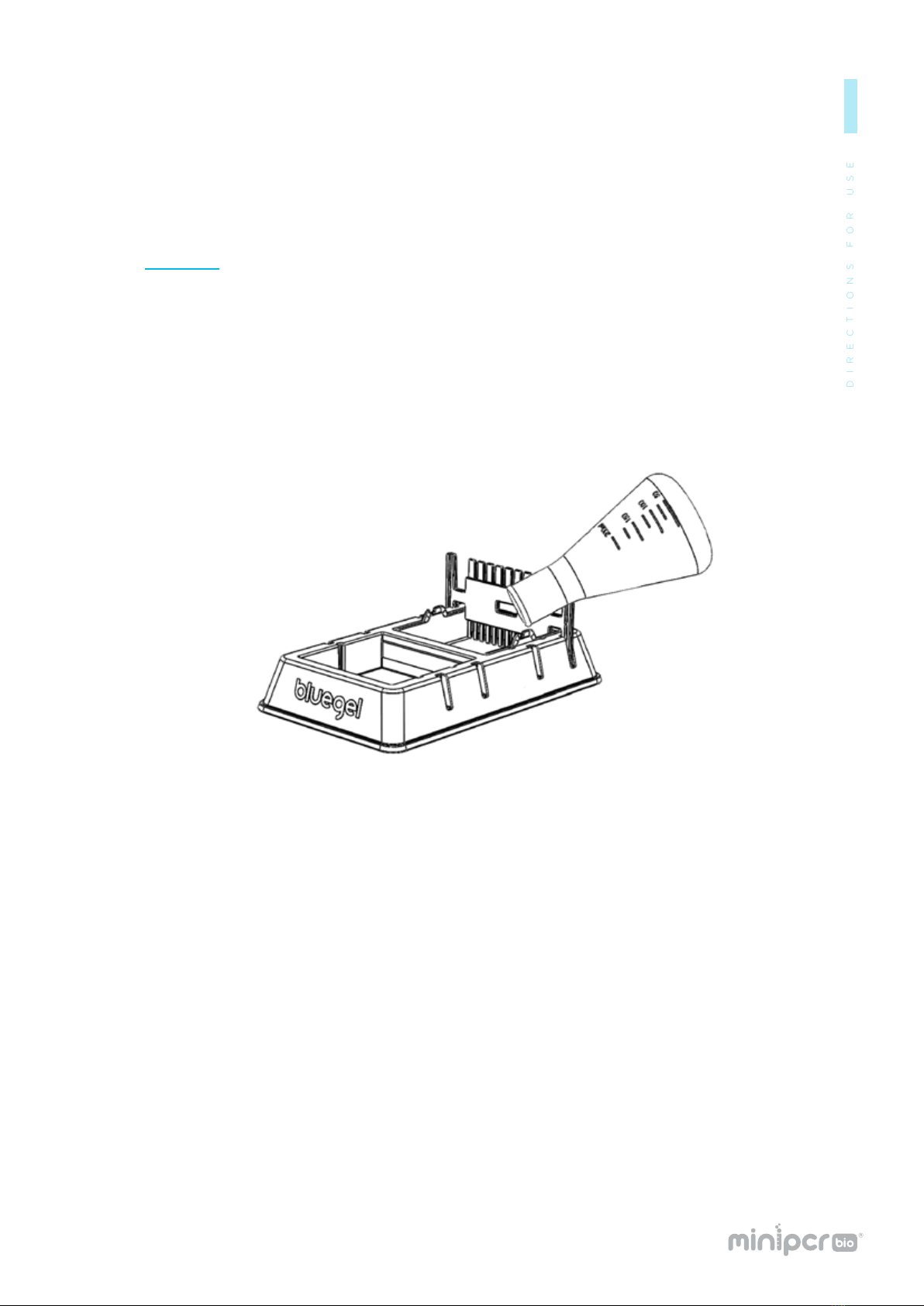
Load the DNA samples in the wells using a micropipette.
9-well combs hold up to 20 μl
13-well combs hold up to 10 μl
Be careful not to puncture the gel with the micropipette tip.
Note: The DNA samples should contain loading dye.
Recommended: To prevent fogging during electrophoresis, spray one pump or less of
ClearView SprayTM inside the orange cover, between the electrodes. Spread to an even
coat using a microber cloth. Wipe gently, do not rub clean.
Place the orange cover on the blueGelTM base. The cover contains the electrodes and
will only fit in one direction, with the (+) electrode positioned to attract the negatively
charged DNA.
Press the power button to start the run. The green LED indicator located next to
the power button should light up. Small bubbles will form near the electrodes.
NOTE: For safety, the power won’t turn on if:
a. The cover is not correctly placed on the base, and electrodes are not making contact
b. There is no buffer in the buffer chamber
c. Using the incorrect buffer (too diluted or too concentrated)
At any time during the run press the lightbulb button to visualize the DNA. The
orange cover filters the excess blue light allowing easier visualization of the fluorescence
emitted by DNA.
1 --
2 --
3 --
4 --
5 --
6 --
7 --