Movesense MD User manual

1
User Guide 2020-12-14 / R78
MOVESENSE MD
User Guide
OP174
2020-12-14 / R78

1
User Guide 2020-12-14 / R78
CONTENTS
CONTENTS................................................................................................................................................................. 1
1
INTENDED USE .................................................................................................................................. 2
1.1
Contraindications ............................................................................................................... 3
1.2
Device description............................................................................................................. 4
1.3
Device lifetime ...................................................................................................................... 5
2
SAFETY..................................................................................................................................................... 6
2.1
Explanation of the markings used on the device ......................................... 6
2.2
Types of safety precautions.........................................................................................7
2.3
Safety precautions..............................................................................................................7
3
GETTING STARTED WITH HEARTRATE/ECG MEASUREMENT .............. 12
4
CARE AND SUPPORT...............................................................................................................14
4.1
Handling guidelines..........................................................................................................14
4.2
Software updates............................................................................................................. 15
4.3
Battery...................................................................................................................................... 15
4.4
Troubleshooting..................................................................................................................17
4.5
Indicator LED ...........................................................................................................................18
5
REFERENCE .................................................................................................................................... 19
5.1
Technical specifications............................................................................................... 19
5.2
Manufacturer ....................................................................................................................... 19
5.3
Compliance..........................................................................................................................20
5.4
Patent notice ...................................................................................................................... 23
5.5
Trademark ............................................................................................................................. 23
5.6
Disposal of device........................................................................................................... 23
5.7
Copyright.................................................................................................................................. 23

2
User Guide 2020-12-14 / R78
1 INTENDED USE
Movesense MD sensor is intended to be used as an ECG and motion signal
measurement device, which sends the measured signals to other devices for
analysis. Movesense MD extracts R-peaks from the recorded ECG signal with
a modified Pan-Tompkins algorithm, allowing R-R interval measurement and
successive heart rate calculation. Movesense MD does not analyze the measured
signals for abnormalities, such as arrhythmias, but sends the signals to the host
device as registered. Thus, Movesense MD does not provide direct diagnosis,
but it provides data for host device to allow further processing. The final signal
interpretation and diagnosis is the responsibility of a certified physician.
Movesense MD sensor does offer programmability and a possibility to run custom
algorithms, which can be developed and verified by the OEM1 3rd party integrator
utilizing the Movesense MD sensor as a component in their system, which the
OEM in question would have separately approved.
•
Movesense MD sensor does not have other user interface than a red indicator
LED, controllable by software, and the signal data is not directly visually readable
from the sensor. A host device, for example a mobile phone or a tablet computer,
is needed to analyze, read and show the measured signal data.
Movesense MD sensor is able to measure the following signals:
-
Single channel ECG waveform
o Sampling frequency: 128/256/512Hz
o Measurement bandwidth: 0.5Hz-40Hz as defined in IEC60601-2-47
o Dynamic range 60mVp-p, max offset: 500mV, resolution: 15 bits
o Heart rate: 20BPM-240BPM, resolution: 1BPM, accuracy: ±1BPM
o R-R intervals: 200ms-2000ms, resolution: 1ms, timing accuracy: ±1ms
▪
Modified Pan-Tompkins algorithm used for R-peak detection
-
Motion (16 bit output resolution)
o Acceleration
▪
±2/±4/±8/±16g, output unit: m/s2, accuracy: ±2%
▪
12.5/26/52/104/208Hz sampling frequency
o Angular velocity
▪
±125/±245/±500/±1000/±2000°/s, output unit: °/s, accuracy: ±2%
▪
12.5/26/52/104/208Hz sampling frequency
o Magnetic field2
▪
±49 gauss, 1.5±10% mgauss /LSB, output unit: mgauss
1 Original Equipment Manufacturer
2 Due to the inherent nature and behavior of the magnetometer measurement circuitry in the vicinity of local
ferromagnetic objects (i.e. the battery), the magnetometer output signal is not linear. The magnetometer is mainly
meant to be used for gyroscope drift compensation in inertial measurement (IMU) application. If the use case
requires absolute magnetic field strength value measurements, an application specific calibration procedure must
be implemented, to the extent considered necessary, in the client application.

3
User Guide 2020-12-14 / R78
-
Additionally, a non-medical temperature measurement capability, which shall not
be used for medical purposes
o Device’s internal temperature
▪
0 to +65°C, accuracy better than ±0.5°C
The Movesense MD sensor has a limited internal recording capability for storing
raw recorded signal data or processed derivatives of the data. This memory can
be utilized by implementing a custom OEM firmware.
As an output of the Movesense MD sensor, the signals are sent by a wireless Bluetooth
connection to a host device for further processing, analyzing and storing, as needed by
the end application.
The Movesense MD sensor is used as an accessory of medical devices.
The Movesense MD sensor may be operated by the patient.
The Movesense MD fulfills the requirements set for the operation in oxygen rich
environment, as specified in IEC 60601-1:2005, 11.2.2.1 b) 1. Movesense MD may
be operated in an oxygen rich environment, when the partial pressure of oxygen is
maintained at or below 85kPa (pO2< 85kPa), equal to an air atmosphere under
a 300kPa overpressure.
1.1
Contraindications
Movesense MD shall not be used as a primary monitoring device for vital
physiological parameters (such as ECG, heart rate, respiratory rate) in clinical
situations where the patient is in an immediate danger, such as during intensive care.
Movesense MD shall not be used as a life sustaining or life supporting device.
Movesense MD may not be used to measure ECG from infants weighing less
than 10kg.

4
User Guide 2020-12-14 / R78
1.2
Device description
Product: Movesense MD sensor
Safety classification:
• Movesense MD is a Class IIa medical device accessory
• Movesense MD is INTERNALLY POWERED EQUIPMENT
• Movesense MD is TYPE BF APPLIED PART, fulfilling the
requirements of the IEC 60601-1 standard
• Movesense MD is a CONTINUOUS OPERATION device
• Movesense MD may be used both in PROFESSIONAL
HEALTHCARE FACILITY ENVIRONMENT and in HOME
HEALTHCARE ENVIRONMENT
• Movesense MD may be used to measure motion, heart
rate and R-R intervals from infants weighing less than 10kg
• The upper limit of the Movesense MD ECG measurement
bandwidth is 40Hz, hence the sensor may not be used to
measure ECG from infants weighing less than 10kg
(as defined in IEC 60601-2-47:2012)
• Movesense MD is suitable for operation in an OXYGEN
RICH ENVIRONMENT
Target users: Medical professionals and consumers. The device may be operated by
the patient.
Device description: Movesense MD is a sensor which is used in connection with
host medical device systems. Movesense MD sensor is a medical device accessory
which records signals for analysis as defined by the medical device manufacturer.
The signal can be ECG waveform or motion. Movesense MD sensor has also a
non-medical temperature measurement capability, which shall not be used for
medical purposes.

5
User Guide 2020-12-14 / R78
1.3
Device lifetime
The maximum expected life of the Movesense MD sensor in normal home use is 7
years. Replace the sensor after this or earlier if
1)
otherwise instructed or
2)
the harsher than normal operating conditions have caused deterioration of the
essential features or
3)
if any damage to the device is observed.
See the section 5.6 for recycling guidance. If any cracks or structural damage is
observed, cease the use and replace the sensor immediately.
NOTE: the battery must be replaced when the sensor does not start or if
the red indicator led does not light up during power-up, when instructed by the
accompanying host application or otherwise when needed. The O-ring and the
sealing surfaces must be visually inspected and cleaned every time the battery
cover is opened, according to the section 4.3.
The maximum expected battery life in the plain heart rate monitoring use case is
400 hours. The maximum expected battery life in the continuous ECG monitoring
use case is 7 days. The maximum expected storage life of the battery before the
first use is 1 year. Always use a fresh battery when a long duration continuous
measurement is anticipated.
The maximum expected service life for the textile heart rate monitor strap is 100
hours of use.
The maximum expected service life for the battery cap O-ring is 10 battery
replacement cycles.
6
User Guide 2020-12-14 / R78
2 SAFETY
2.1
Explanation of the markings used on the device and in the documentation
Manufacturer
Date of manufacture
CE marking and the notified body identity number
WEEE Directive logo. Do not throw in the garbage
LLeft side electrode connection
R
Right side electrode connection
See user guide for important information
Type BF applied part
Bluetooth logo. The sensor utilizes a Bluetooth LE radio for wireless
communications
Fragile, handle carefully
Keep away from sunlight
Operating temperature range
Operating humidity range
Operating pressure range

7
User Guide 2020-12-14 / R78
Machine wash 30°C / 86°F
Do not tumble dry
Do not iron
Do not bleach
Do not use fabric softeners
2.2
Types of safety precautions
WARNING: is used in connection with a procedure or situation that may
result in serious injury or death.
CAUTION: is used in connection with a procedure or situation that will
result in damage to the device, affect the measurement results or pose a risk to
the safety of the patient/user or the operator.
NOTE: is used to emphasize important information, which the user and the
operator must be aware of to guarantee safe and practical use.
TIP: is used for extra tips on how to utilize the features and functions of the
device.
2.3
Safety precautions
WARNING: Only for intended use.
WARNING: The Movesense MD sensor must not be used for purposes
other than what it is intended for.
8
User Guide 2020-12-14 / R78
WARNING: Stop the usage immediately if the sensor is damaged or if a
change in the performance is observed.
WARNING: Stop the usage immediately if an allergic reaction is observed.
WARNING: Do not modify this equipment without prior written authorization
of the manufacturer. If this equipment is modified, appropriate inspection and
testing must be conducted to ensure continued safe use of the equipment.
WARNING: Always consult your doctor if you have a medical condition and
before beginning an exercise program. Overexertion may cause serious injury.
WARNING: Always consult your doctor before using the sensor if you have
a pacemaker or other implanted device. Although several implanted pacemaker
manufacturers state the risk associated with the simultaneous use is low, it
is essential to consult a doctor who knows the exact type and model of the
implanted device in question before using the sensor. In any case keep the sensor
at least 15cm/6” away from the implanted device.
WARNING: Do not use the sensor during magnetic resonance imaging (MRI),
unless specifically approved by the personnel operating the MRI equipment.
The coin cell battery inside the device is magnetic.
WARNING: Not to be worn by multiple users if consequences from
possible cross contamination may be severe. Careful cleaning and disinfection is
recommended to prevent cross infection if worn by multiple users.
WARNING: The conductive parts of the sensor and/or electrode
connections must not be allowed to contact any conductive parts, including
protective earth connection.
WARNING: Keep the sensor and any accessories away from the reach of
children, pets or pests when not in use.
WARNING: The battery used must be compliant with the requirements of
the IEC 60086-4 lithium battery safety standard.
WARNING: KEEP THE BATTERY OUT OF REACH OF CHILDREN.
EVERY EFFORT MUST BE TAKEN TO PREVENT ACCIDENTAL SWALLOWING OF THE
BATTERY OR OTHER PARTS. IF ACCIDENTAL SWALLOWING IS SUSPECTED, SEE
DOCTOR IMMEDIATELY. THE BATTERY TYPE IS LITHIUM
/ MANGANESE DIOXIDE (Li/MnO2).

9
User Guide 2020-12-14 / R78
WARNING: Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no closer than 30
cm (12 inches) to any part of the Movesense MD, including cables specified by
the manufacturer. Otherwise, degradation of the performance of this equipment
could result.
WARNING: Use of this equipment adjacent to or stacked with other
equipment should be avoided because it could result in improper operation.
If such use is necessary, this equipment and the other equipment should be
observed to verify that they are operating normally.
WARNING: Do not use the sensor with accessories or parts not meant for it
or interconnect with other equipment that are not intended to be interconnected
with it, as the result may be unsafe and may negatively affect the electromagnetic
compatibility.
CAUTION: Do not apply solvent of any kind to the product, as it may
damage the surface.
CAUTION: Do not use the sensor on patient skin during defibrillation.
CAUTION: Do not use on patient skin simultanously with HF surgical
operation.
CAUTION: Do not apply insect repellent on the product, as it may damage
the surface.
CAUTION:Do not knock or drop the product, as it may get damaged.
CAUTION: Do not modify the device. Any modifications are potentially
unsafe.
NOTE: If the storage temperature is below -20°C /-5°F, allow the device’s
internal temperature to stabilize for 10min before use.
10
User Guide 2020-12-14 / R78
NOTE: The sensor is immediately usable when brought to room
temperature from a storage temperature of -20°C to +60°C/-5°F to +140°F
-20°C
+60°C
0% RH
99% RH
300hPa
3000hPa
NOTE: If the Movesense MD sensor is used for heart rate measurement, the
standard Heart Rate Service may be used, as specified by the Bluetooth SIG3,
in connection with a compatible general purpose host application or device. If
extended functionalities like ECG or motion measurement are used, a dedicated
host application is required, capable of receiving the custom data.
NOTE: Use at least 30cm/12” away from the sources of power line
frequency magnetic fields, radio frequency communcations equipment and other
sources of radio frequency signals (such as radars or microwave ovens).
If the measurement results are fluctuated by a strong nearby radio frequency
disturbance source, move further away from the source of the radio frequency
disturbances.
To avoid any degrading effects of the external electromagnetic disturbances, the
sensor should be used in connection with equipment fulfilling the IEC60950 and/or
EN60601-1 standards. Avoid using the sensor in the proximity of
3 For details see www.bluetooth.org. The Bluetooth LE radio technology used in the Movesense MD
is specified in the Bluetooth v4.0 specification. Suitable host devices include mobile phones, tablet
computers and other devices compliant with the Bluetooth v4.0 (or above) specification and running a
suitable host application capable of processing the measured signals.
For plain heart rate monitoring use case utilizing the in Bluetooth LE Heart Rate Service, capable of providing
heart rate and R-R intervals, as specified by the Bluetooth SIG, a suitable sports watch can be used. An
example of a such a device is Suunto S9 sports watch.

11
User Guide 2020-12-14 / R78
electrostatic disturbance sources. Do not use close to a 2.4GHz signal source, as
strong signal may negatively affect the performance of the Bluetooth radio link.
NOTE: The Movesense MD sensor is waterproof and can be used in
wet environments. The IP68 ingress protection rating means that the sensor
withstands submerging to a depth of 1m/3.3ft underwater for a duration of one
hour.
It must be taken into account that the Bluetooth connection will be interrupted
if a large enough RF energy absorbing body of water is inserted between the
Movesense MD sensor and the respective host device.
NOTE: When the sensor is not in use, do not allow the two metal studs
to simultaneously contact an electrically conductive medium. If the studs are
connected, for example via a metal surface or a moist fabric, the sensor will
remain powered on and this will unnecessarily consume the battery.
12
User Guide 2020-12-14 / R78
3 GETTING STARTED WITH HEART RATE/ECG
MEASUREMENT
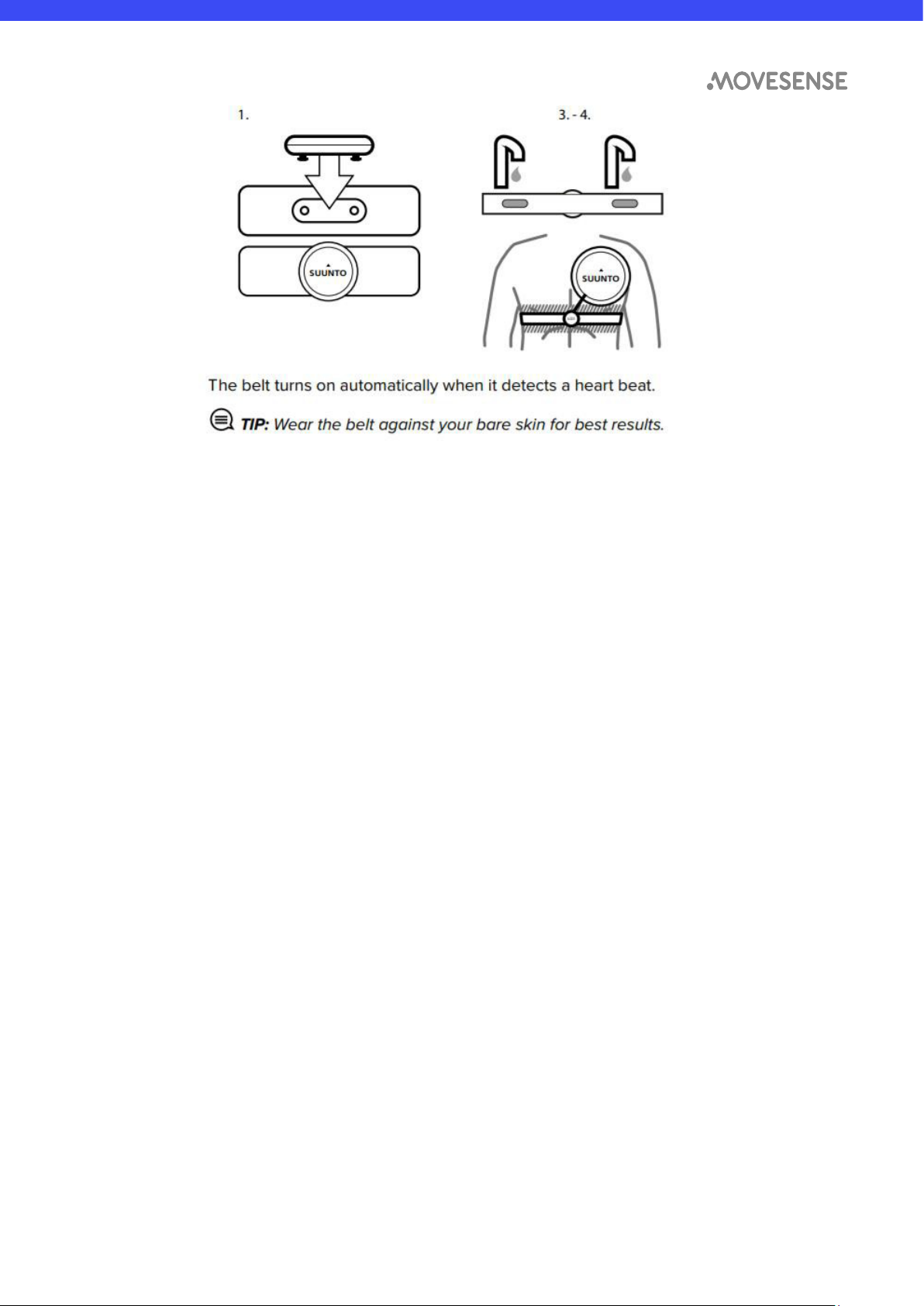
To start using the Movesense MD sensor with a heart rate belt4:
1. Snap the sensor firmly into the belt connector. Make sure that the electrode
connection marked with “L” is on the left side of the patient and the
electrode marked with “R” is on the right side of the patient.
2. Adjust the belt length as needed.
3. Moisten the belt electrode areas with water or electrode gel.
4. Put the belt on so that it fits snugly and the logo on the front face of the sensor
is facing up. The sensor turns automatically on upon detecting electrical signal.
CAUTION: If the sensor is worn upside down and if recording ECG, the
measured raw ECG signal is inverted.
CAUTION: If the electrical connection to the user’s body is poor, the
measured ECG signal will be attenuated.
WARNING: The Movesense MD sensor contains an additional internal
functionality to speed up the recovery from an excessively high ECG input
overvoltage, such as a static discharge. In case there is an excessively large
amplitude input, larger than 100 times a typical QRS complex, the ECG channel
is briefly disconnected from the patient and the sensor runs through a dedicated
reset procedure to keep the AC-coupled ECG signal within its measurement
range. After the automatic reset process is completed, the ECG signal path is
connected to the patient again, and the ECG measurement continues normally.
This ECG input reset process may take up to 1.5 secods to complete, during
which time the patient ECG channel shows the reset pulse instead of the patient
ECG.
WARNING: Be careful to keep the heart rate monitor strap from getting
snagged into external objects, as a choking hazard could develop.
4 Compatible heart rate belts are available separately: Movesense strap, order code: SS050114000
(30-pack/size M)
13
User Guide 2020-12-14 / R78
Pairing
You need to connect (pair) your Movesense MD sensor with compatible Bluetooth®
Low Energy (BLE) devices to view the measurement data. These devices can be, for
example mobile devices running respective host applications for data visualization.
Pairing procedures may vary, so refer to the instructions of your mobile application
for guidance.
You can pair the sensor with multiple host devices, but only one connection can be
active at a time.
Follow the usage guidance provided by the host application.
The sensor turns automatically off, if no electrical signal is detected within a
predetermined time and the sensor is not connected to a Bluetooth host device.
The maximum continuous ECG recording time with 256Hz sampling rate and a fresh
battery is 7 days.
The heart rate is calculated using the R-R intervals: HR [BPM] = 60000/R-R [ms]
14
User Guide 2020-12-14 / R78
4 CARE AND SUPPORT
4.1
Handling guidelines
Movesense MD sensor module should be rinsed clean with fresh water after each use.
If more thorough cleaning is needed, the sensor may be quickly wiped with a soft cloth
moistened with ethanol based disinfectant5. No immersion in chemicals other than
water is allowed.
CAUTION: Do not pull the sensor module straight off the connector.
This may damage the belt connectors. Unsnap one side at a time.
The belt should be machine washed in 30° C, preferably using a wash bag, after
every 2-3 uses. See the belt tag for further washing instructions. Replace the belt
every 100 hours, or sooner, if deterioration in performance or physical properties
is observed.
Cleaning and disinfection of the sensor as well as washing the strap can be
performed by the device operator or the patient/user.
CAUTION: Do not machine wash the sensor module. Machine washing
damages the module.
WARNING: Careful cleaning and disinfection by the operator is
recommended between uses to prevent cross infection if worn by multiple users
or patients. Disinfect before and after each use. Allow disinfectant to dry before
taking into use. Not to be worn by multiple users if consequences of cross
contamination may be severe.
NOTE: Repetitive disinfection with ethanol based disinfectant may in the
long run cause aging and discoloration of the plastics used. Discoloration does
not affect the safe use. If any cracks or structural damage is observed, replace
the sensor.
CAUTION: Long term continuous usage of the belt may cause irritation.
Cleaning and disinfection is recommended to prevent long term irritation and
infection. Be extra cautious in high temperature and/or humidity.
CAUTION: The maximum allowable continuous skin contact time in >43°C
ambient temperature is 1 hour. Exercise caution when touching or using the
Movesense MD sensor in skin contact in elevated ambient temperatures.
In case the Movesense sensor is placed on the body in elevated ambient
temperature, it is recommended to equalize the surface temperature of the
Movesense MD sensor with that of the user’s body by briefly holding the sensor
5 Minimum ethanol content: 70 w-%. Berner A12T equivalent recommended.
15
User Guide 2020-12-14 / R78
in a closed palm, before placing it on other more sensitive parts of the body.
NOTE: Store in a dry cool place and away from the sunlight between uses.
NOTE: Contact the manufacturer in case assistance in needed in setting
up, using or maintaining the device or to report unexpected operation or events.
If the sensor is an OEM variant, please contact the OEM that supplied the sensor,
according to the separate instructions provided by the OEM in question.
4.2
Software updates
The Movesense MD sensor firmware can be updated over Bluetooth.
Please refer to the instructions of your host application for guidance.
4.3
Battery
The Movesense MD sensor uses a 3-Volt lithium coin cell battery (CR 2025).
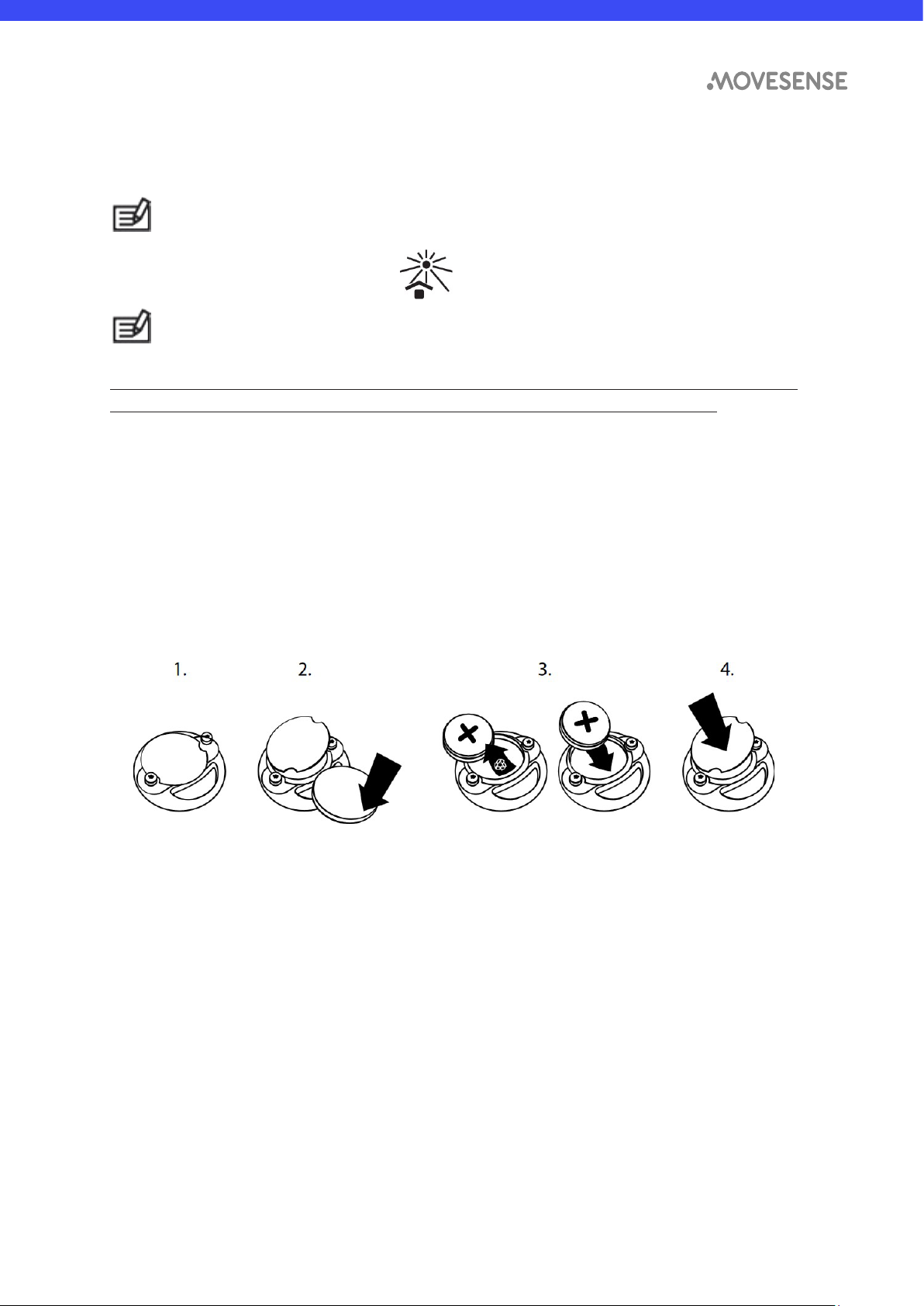
To replace the battery:
1. Remove the sensor from the Movesense connector.
2. Open the battery cover using a coin as a tool.
3. Replace the battery by inserting the replacement battery first into the battery
cover, positive side up, and then pressing the sensor body on the battery
cover. Make sure that the O-ring is in correct position in the groove on the
battery cover before closing the battery cover. Please dispose of the old
battery according to the local rules and legislation, treating it as battery waste.
Do not throw it in the garbage.
4. Firmly close the battery cover. Make sure that the O-ring is not visible after
closing the battery cover.

16
User Guide 2020-12-14 / R78
NOTE: Inspect battery compartment carefully for any leakage or residue
from the old battery. If residue exists, replace the sensor. The battery must be
removed prior to long term storage.
NOTE: Visually inspect the battery contacts, O-ring and the sealing surfaces
for contamination. Remove any contamination and clean with a dry soft non-
clogging cloth. Replace the O-ring if damaged6. Replace the sensor if sealing
surfaces are damaged.
NOTE: The battery is to be replaced if the accompanying host application
instructs to do so, if the sensor does not power up or if the red indicator led does
not light at power up normally.
NOTE: Make sure that the plastic insulator under the battery is intact and in
place when replacing the battery.
6 O-ring size: 20.3mm x 0.9mm, Silicone, shore A 70
17
User Guide 2020-12-14 / R78
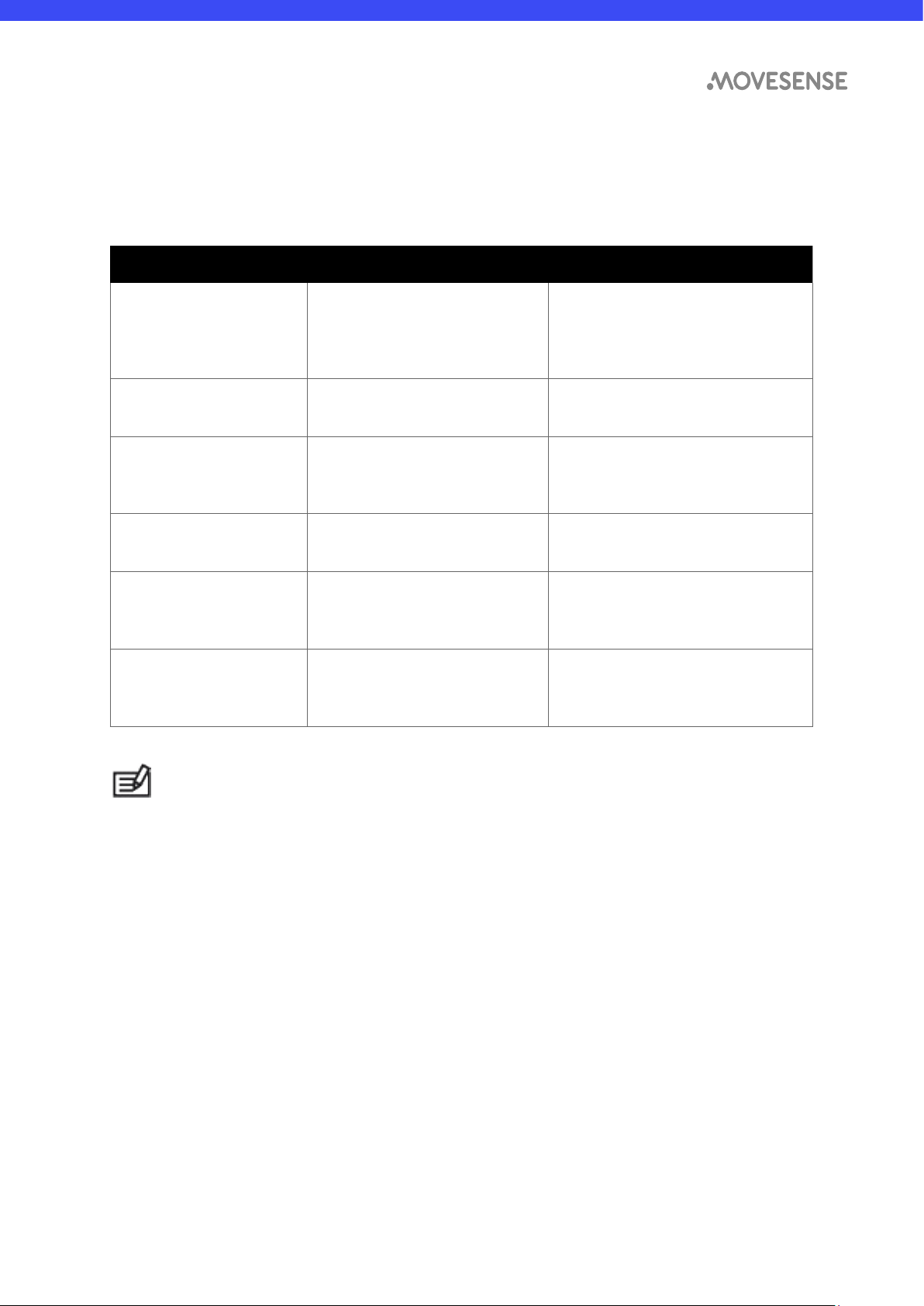
4.4
Troubleshooting
The device does not turn on
automatically upon becoming in
contact with the patient
Battery empty Replace the battery
according to the
instructions
ECG signal level is low or the
signal quality is low
Patient connection is dry, strap
is contaminated dirty
Moisten the contacts,
wash the strap, replace
the strap
Sensor or strap is damaged Mechanical damage Replace the sensor or strap
No connection to the host
application or device
No mobile application installed
or Bluetooth not enabled on
the host device
Consult the application
specific user guide
for the application
installation and usage.
Turn on the Bluetooth
radio in the host device.
Signal lost when the sensor
is too far away from the host
device
Signal attenuated Bring the host device
closer to the terminal
Signal is inverted Sensor attached upside down Re-attach the
sensor observing
the correct
orientation
Sensor cannot be connected to
a host device
Sensor already connected to another
host device
Non-compatible host device
Use the sensor with one
device at a time only
Use a host device
complying with
Bluetooth
4.0 or above
18
User Guide 2020-12-14 / R78
4.5
Indicator LED
Movesense MD houses a red color indicator led on the top edge of the sensor
housing, visible through the plastic casing. The functionality of the indicator led is as
follows:
On for 2s when the
device turns on
The device turns on and the LED
functionality is tested.
Normal operation
None
Off during the normal
use
Normal operation None
2-7 brief flashes Normal operation; battery level
measurement is underway
None
Continuous rapid
flashing
The battery is empty Stop the usage and replace the
battery
LED constantly on The sensor is in firmware update
mode
Follow the firmware updating
instructions on the accompanying
application
LED does not turn on
when the sensor is
started
The battery is empty Replace the battery
NOTE: the OEM integrator may modify the functionality of the indicator LED
to suit the application specific needs. Therefore the OEM application specific user
guide must be consulted for possible additional information.
19
User Guide 2020-12-14 / R78
5 REFERENCE
5.1
Technical specifications
•
Device name and type identifier: Movesense MD sensor module, OP174
•
Weight: 9.4 g/0.33 oz (battery included)
•
Diameter: 36.5 mm/1.44 in
•
Thickness: 8 mm/0.32 in
•
Operating conditions: -20°C to +60°C/-5°F to +140°F, 0-99%
Relative Humidity, Pressure: 300hPa to 3000hPa
•
Storage and transportation conditions: -30°C to +60°C/-22°F to +140°F,
0-90% Relative Humidity, non-condensing, Pressure: 700hPa to 1060hPa
•
Water resistance: 30 m/100 ft (tested according to ISO 6425
standard), IP classification: IP68 (1m/1h)
•
Battery type: Maxell CR2025 Lithium / Manganese Dioxide (Li/MnO2)
o The battery used must fulfill the requirements of the IEC60086-4 safety
standard
•
Radio technology: Bluetooth Low Energy (BLE)
•
Transmission frequency: 2.400GHz - 2.4835GHz, Modulation: GFSK, Channel
bandwidth: 1MHz, Pmax = 0dBm, ERP = -4.85dBm
•
GMDN number: 12391 Wearable multiple physiological parameter recorder
5.2
Manufacturer
Suunto Oy
Tammiston kauppatie 7 A
FI-01510 Vantaa FINLAND
www.movesense.com
The time of manufacturing is included in the device’s
serial number, as manufacturing year and week.
Example: serial number 195012356789:
Manufactured during week 50 of year2019
This manual suits for next models
1
Table of contents
Popular Accessories manuals by other brands

3dsimo
3dsimo KIT 2 Assembly manual

Magapor
Magapor Z-450 instruction manual

PCB Piezotronics
PCB Piezotronics 201B01 Installation and operating manual
Guardian
Guardian Germ Guardian LW9 Use & care instructions

DirekTronik
DirekTronik LDS02 user manual

Meltem
Meltem M-WRG-II FSF Installation instructions and user guide











