NEURO20 N20 PRO-SYS User manual
© 2022 Neuro20 Technologies®
NEURO20 PRO SYSTEM
OPERATING MANUAL
Version 1.0
Electro Muscle Stimulation

Neuro20 PRO System Operating Manual
© Neuro20 Technologies 2022
No parts of this product may be reproduced, sold, or distributed in any form without the written
permission of the publisher.
® Indicates a trademark is registered in the U.S. and other countries.
Published by Neuro20 Technologies Corp.
3802 Spectrum Blvd. Suite 116E
Tampa, FL 33626 USA
+1.917-503-6876
www.neuro20.com
Copyrighted in the United States of America
Product No. Neuro20 PRO System 1.0 Operating Manual: N20PRO-OM-V1.0 07/22
This Neuro20 PRO System belongs to:
_______________________________________________
Owner
_______________________________________________
Address
_______________________________________________
City, State/Province, Country
_______________________________________________
Telephone

Neuro20® PRO System
OPERATING MANUAL
N20PRO-OM-V1.0 07/22
®
© NEURO20 TECHNOLOGIES 2022

ii | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22

www.neuro20.com | iii
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
TABLE OF CONTENTS
SYMBOLS DESCRIPTION................................................................IV
INTRODUCTION............................................................................... 1
INDICATIONS OF USE.......................................................................1
SAFETY.............................................................................................2
CONTRAINDICATIONS......................................................................2
WARNINGS.......................................................................................2
PRECAUTIONS.................................................................................5
ADVERSE REACTIONS.....................................................................8
PRODUCT DESCRIPTION.................................................................9
SYSTEM COMPONENTS AND OVERVIEW......................................11
Neuro20 PRO Control Box.......................................................12
Battery & Charger.....................................................................13
Operating Tablet ......................................................................14
Neuro20 Smart Suit .................................................................15
OPERATING PROCEDURES............................................................17
ELECTROMAGNETIC COMPATIBILITY............................................48
TECHNICAL SPECIFICATIONS........................................................53
TROUBLESHOOTING......................................................................55
GENERAL MAINTENANCE..............................................................60
PRODUCT REGISTRATION..............................................................64
LIMITED WARRANTY.......................................................................64
IV | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
The symbols and their descriptions will appear throughout sections of the
Operating Manual. These symbols are associated with the Contraindications,
Warnings, Precautions, and potential Adverse Effects. When seeing a symbol
read carefully and consider the information prior to operating the equipment.
SYMBOLS DESCRIPTION
Symbol Description
CAUTION
The “CAUTION” symbol indicates text that will explain possible safety
hazards that could potentially cause injury or damage to equipment.
DANGER
The “DANGER” symbol indicates potential imminent hazardous safety
situations that could result in death or serious injury.
EXPLOSION HAZARD
The “Explosion Hazard” symbol indicates possible safety
hazards if this equipment is used in the presence of
ammable materials.
DANGEROUS VOLTAGE
The “Dangerous Voltage” symbol indicates possible hazards resulting
in the electrical charge delivered in certain program congurations of
waveforms.
BIOHAZARDOUS MATERIALS
The “Biohazard” symbol indicates possible hazards resulting from the
improper handling of components and accessories that have come in
contact with bodily uids or components that need proper disposal.
NON-IONIZING ELECTROMAGNETIC RADIATION
The “Non-ionizing Electromagnetic Radiation” symbol indicates
possible hazards resulting from elevated, potentially dangerous levels
of non-ionizing radiation.
KEEP DRY
The “Keep Dry” symbol indicates possible hazards resulting when the
suit is operated in water.
BF COMPONENTS
“BF Components” are devices with conductive contact to the User.

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 1
Congratulations on your purchase of the Neuro20 PRO System.
Neuro20 Technologies suggests that operators learn the contents of this manual.
Owners must register the manufacturing number of the product online at
www.neuro20.com. The system will only be activated following product
registration.
Neuro20 Technologies suggests that the manual is kept with the system and
stored in a safe place. The manual is also available at www.neuro20.com.
Do not operate the system if there is any possibility of damage.
If damage is a concern, rst refer to Troubleshooting (pg.56). If
troubleshooting does not resolve the concern, please contact
+1.917-503-6876. Proper use and maintenance of the system is the
sole responsibility of the registered owner/operator.
The Neuro20 PRO System is a medical device not intended for resale, loan,
or lease to any third-party operator. Any resale, lease, loan, or distribution of
the Neuro20 PRO System may only occur with the written consent of Neuro20
Technologies. If authorized consent is obtained, then the new owner must re-
register the device with the company.
Caution! Federal Law restricts this device to sale by or on the
order of a practitioner licensed by the law of the State in which
he/she practices to use or order the use of this device.
Note! When increasing stimulation, 1% equates to a 2 mA current.
INTRODUCTION
The FDA approves electrical stimulation for the following indications of use:
Muscle Re-Education
Maintain or Increase Range of Motion
Increase Local Blood Circulation
Relaxation of Muscle Spasms
Prevention of Muscle Atrophy
Improve Muscle Strength
INDICATIONS OF USE

2 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
Pacemaker
Powered muscle stimulators should not be used on patients with cardiac
demand pacemakers.
SAFETY
Contraindications, Warnings, Precautions, Potential Adverse Effects
CONTRAINDICATIONS
WARNINGS
Long Term Effects
The long-term effects of electrical stimulation are unknown.
Application of Electrodes to Other Body Locations
Stimulation should not be applied over the carotid sinus nerves,
particularly in patients with a known sensitivity to the carotid sinus. Stimulation
should not be applied across or through the head (transcerbally), directly on
the eyes, covering the mouth, on the front of the neck, (especially the carotid
sinus). Severe spasm of the laryngeal and pharyngeal muscles may occur
if placed on the neck and the contractions may be strong enough to close
the airway or cause difculty in breathing. Stimulation should not occur from
electrodes placed on the chest and the upper back or crossing over the heart
(transthoracic) in that the introduction of electrical current into the heart may
cause cardiac arrhythmias. Note: The pectoralis and the complex of back
muscles are supercial and electrical stimulation to these muscles is not
considered trans-thoracic.
Fever/Infection /Acute Inammation
Stimulation should not be applied over swollen, infected, or inamed
areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose
veins, etc.
Active Cancer
Stimulation should not be applied over, or in proximity to, cancerous
lesions. Electrical stimulation should not be applied directly over an area of the
body where malignancy is known to be present.
Implanted Debrillator
Do not use electrical stimulation on patients with an implanted debrillator,
because this may cause electric shock, burns, electrical interference, or death.
Device User Manual
Do not operate this device until the User Manual with the Indications of Use,
Contraindications, Warnings, Precautions, and potential Adverse Effects are
carefully read and understood. If there are any questions, contact Neuro20
6876 prior to use.
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 3
WARNINGS
Implanted Medical Device
Do not use electrical stimulation on patients who have an implanted metallic
or electronic device because this may cause electric shock, burns, electrical
interference, or death.
Patient Cognition / Cooperation/Children
Do not apply if the patient does not understand the potential risks of treatment.
Muscle Breakdown or Bruising coupled with Delayed Onset Muscle Soreness
Over working a muscle can result in some muscle breakdown. This condition
results in muscle ber disruption and small muscle cellular contents, such as
myoglobin, exits the ber and can appear as a bruise. Myoglobin also enters
the blood stream is eventually cleared by the liver and kidneys. Urine can
appear quite dark as the myoglobin clears. Discontinuation of the Neuro20
system and a review by a physician is important if this occurs. Resuming
treatment requires clearance by a physician. Reducing the intensity of
stimulation in future sessions and reducing overall exercise is warranted.
Delayed Onset Muscle Soreness may also be noted without signs of muscle
breakdown. This uncomfortable experience is very often noted after the
rst few sessions of electrical stimulation coupled with exercise. It can also
be noted with increased exercise alone. Please review the physiology of
DOMS. Soreness does not mean that stimulation should be discontinued,
BUT reducing the intensity, and reducing the concurrent exercise is important
as your treatment continues. If DOMS continues for more than a few days
following electrical stimulation and exercise, then discuss this with your
healthcare practitioner. We suggest waiting until the soreness is eliminated, or
markedly reduced, before continuing use.
Skin Preparation
Apply electrical stimulation only to normal, clean, healthy skin.
Pregnancy
Do not apply electrical stimulation over the lumbar or abdominal region, or over
the uterus during pregnancy (to prevent uterine contraction).
Precaution: Safety of powered muscle stimulators for use during pregnancy
has not been established.
Menstruation
Do not use the Neuro20 electrical system over the lumbar or abdominal regions
or over the uterus during menstruation as stimulation may temporarily increase
menstrual ow.
Reproductive Organs
Do not apply electrical stimulation treatment over the testes. Electrical
stimulation may affect organ function.
(continued)

4 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
WARNINGS
DVT / Thrombophlebitis
Neuromuscular electrical stimulation should not be applied directly over
or near Deep Vein Thrombosis (DVT) since it activates muscles causing
contractions. This should be avoided in areas following an acute DVT when
the thrombosis is not completely resolved. Follow treating physician guidelines
on recommended activity levels and stimulation use. If the patient or subject is
not permitted exercise, NMES therapy should be avoided.
Note: Generally, NMES over a DVT of six weeks or less should be avoided
altogether.
Cardiac Disease
Only low intensities and short treatment times should be used since
stimulation of practically any afferent autonomic nerve (especially the Vagus
nerve) in the body may cause a change in cardiac rate. Note: Consult with the
patient’s physician before using electrical stimulation because the stimulation
system may cause lethal rhythm disturbances to the heart in susceptible
individuals.
Medical Equipment
Simultaneous connection of a patient to a high frequency surgical medical
equipment may result in burns at the site of the stimulator electrodes and
possible damage to the
stimulator.
Diathermy/Microwave
Operation in close proximity (e.g.1m) to a shortwave or microwave therapy
medical equipment may produce instability in the stimulator output.
Application of electrodes near the thorax may increase the risk of cardiac
brillation.
Monitoring Equipment
Electrical stimulation should not be applied to patients connected to patient
monitoring equipment, as the simulation may inuence the proper operation of
the monitoring equipment.
Explosion hazard exists if the Neuro20 PRO System is used in the presence
of ammable anesthetics mixture with air, oxygen, or nitrous oxide.
External Stimulator Systems
Electrical stimulation should not be applied directly over external stimulator
systems with lead wires.
To safely terminate operation of this device, press the red STOP button on the
Neuro20 PRO Control box.
(continued)

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 5
WARNINGS
User Activity
Portable powered muscle stimulators should not be used while driving,
operating machinery, or during any activity in which involuntary muscle
contractions may put the user at undue risk of injury. Do not use when bathing
or swimming. Do not apply powered muscle stimulators while falling asleep.
No modication of this equipment is allowed. Modication of the equipment
may cause improper functioning which could lead to injury or death.
(continued)
PRECAUTIONS
Epilepsy
Caution should be used in persons with suspected or diagnosed epilepsy
or seizures. Patients with suspected or diagnosed epilepsy should follow
precautions recommended by their physicians.
Healing Bones
Caution should be used with electrical stimulation when there is a tendency
to hemorrhage following acute trauma, or fracture, in the presence of recent
surgical procedures, or healing bone and soft tissue when muscle contraction
may disrupt the healing process. Caution should be used when applying
electrical stimulation over areas of the body which lack normal sensation.
Absent or diminished sensation areas should be avoided or, if needed, to
be treated with caution. Always determine acceptable intensity levels for
desensitized areas that are likely to be less than intensity levels tolerated on
normal skin in the opposite or related body parts.
Hypersensitivity
Some patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrically conductive medium. The irritation can
usually be reduced by using an alternate conductive medium, or alternate
electrode placement. Slightly increase electrode hydration and /or add normal
saline spray to improve conductance. Adjusting the suit electrode placement
may also reduce hypersensitivity.
Prescribing Practitioner
Electrode placement and stimulation settings should be based on the
guidance of the prescribing practitioner.
Children or Unqualied Persons
Powered muscle stimulators should be kept out of reach of children. Powered
muscle stimulators should be kept out of reach of unqualied persons.
6 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
PRECAUTIONS
Lead Wires
Never connect lead wires to a power line or electro-surgery equipment.
Powered muscle stimulators should be used only with the leads and
electrodes recommended for use by the manufacturer.
User Activity
Portable powered muscle stimulators should not be used while driving,
operating machinery, or during any activity in which involuntary muscle
contractions may put the user at undue risk of injury. Do not use when bathing
or swimming. Do not apply powered muscle stimulators while falling asleep.
Output Intensity
Gradually increase the output intensity (power for each electrode) to the
desired level, or to user tolerance, while monitoring the Operating Tablet
display and asking for verbal feedback from the patient. To prevent startling
do not apply greater power than tolerated. The output intensity should be
increased gradually as user responses may vary greatly.
Conductive Mediums
An appropriate amount of coupling water in the electrodes and on the skin is
important to ensure safe and optimal energy transmission to the tissue. Use
of hand or body lotions or gels or ultrasound gels are not appropriate for use
with the Neuro20 system and may temporarily or permanently interfere with
stimulation function.
Bleeding Tendency
Use caution with electrical stimulation when a patient tends to bleed internally,
such as following an injury or fracture.
Treatment Monitoring
Treatment areas should be self-checked before and after application, and if
there is evidence of pain or irritation, adjust the output lower until it is tolerated.
Medicated Patches, Salves, Creams
The effect of electrical stimulation may be altered by the presence of these
materials applied to the skin.
Hot / Cold Packs
Caution is recommended when treatment follows the application of hot or
cold therapy, which may alter user sensation. Application of thermal agents
over areas of impaired circulation should be performed with caution as the
circulation may be insufcient to heat or cool the tissue, altering the patient’s
perception of warmth and pain.
(continued)

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 7
PRECAUTIONS
Skin Inspection
Inspect and cleanse the skin prior to application. Following treatment, check
the skin for evidence of irritation and if present, treat as appropriate. If there is
skin irritation following treatment, shorten treatment time at the next treatment
and/or reduce intensity, and if necessary, discontinue use.
Service / Repair Shock Hazard
A potential electric shock hazard exists once the device’s outer casing is in
part or fully removed. Only qualied service personnel should perform service
and repairs. Do not Tamper with or remove the outer casing.
Cleaning
The Control Box must be disconnected from the Smart Suit before washing.
When cleaning the electronic Control Box never immerse or wash with water
or other liquids. Avoid oil, water, metal, or foreign substances to penetrate the
battery compartment, charger, Control Box, or suit connection.
Condensation
Sudden temperature changes can cause condensation to build up inside
of the stimulator, allow for the Neuro20 PRO Control Box to reach ambient
temperature before use.
Strangulation can occur due to length of exposed component materials. Do not
wrap any exposed component part around the throat or neck area. Keep out of
the hands/reach of children at all times.
The Neuro20 PRO System contains small parts that will be harmful if
swallowed and no part or component is intended for human consumption.
Seek medical attention if swallowed.
Care must be taken when operating this equipment around other equipment.
Potential electromagnetic or other interference could occur to this or to
the other equipment. Try to minimize this interference by not using other
equipment in conjunction with it.
Electronic monitoring equipment (such as ECG monitors and ECG alarms)
may not operate properly when electrical stimulation is in use.
Do not operate this unit in an environment where other devices are being used
that intentionally radiate electromagnetic energy in an unshielded manner.
(continued)

8 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
ADVERSE REACTIONS
Skin irritation and burns beneath the electrodes have been reported with
the use of powered muscle stimulators. Discontinue use and treat appropriately if this
occurs. Lower the intensity of the stimulation during any subsequent session.
Reporting Adverse Reactions
Neuro20 encourages all patients to report any adverse reactions to their healthcare
adverse reaction reports in order to maintain updates, transparency, and public safety at all
times and only share information to meet any necessary regulatory requirements.
Medical electrical equipment needs special precautions regarding EMC.
Portable and mobile RF communication equipment can be affected by other
medical electrical devices. If you believe interference is occurring, please
consult the ELECTROMAGNETIC COMPATIBILITY section to assist in
removing the interference.
Common RF emitting devices and electromagnetic security systems (cellular
phones, two-way radios, cordless phones, paging transmitters, RFID devices,
etc.) may interfere with the operation of the Neuro20 PRO System. The
Neuro20 PRO System has been tested in the presence of these types of
devices and while no adverse event occurred, the device should not be
operated within the vicinity or environment as another RF emitting device.
PRECAUTIONS (continued)

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 9
The Neuro20 PRO System is a powered muscle stimulator designed for
individual or group rehabilitation and recovery.
The system can create co-contraction muscle resistance as well as optimized
sequenced movement patterns. The involuntary muscle activation can be
voluntarily over-ridden through intentional exercise. This promotes power,
endurance as well as coordination. It increases circulation needed for recovery
with limited impact on ligaments, tendons, and joints, utilizing stimulation
patterns. Individual intensity levels can be modulated for each muscle group.
One to ten patients may be treated within a session.
The Neuro20 PRO System is a wearable textile and supporting software
platform, that provides electrical muscle stimulation interventions. The system
utilizes electrical stimulation to create a motor neuron recruitment of muscle
ber (involuntary contraction), thereby bypassing the neural pathway that
occurs during voluntary muscle recruitment. When combined with a voluntary
movement, the contractions create enhanced performance and recovery.
Users may be actively engaged within a variety of training modes while the
clinician/operator is controlling the software; or a User can receive therapeutic
intervention at a respective physical rehabilitation facility. The system will actively
recruit multiple muscle bers per repetition and enhance movement patterns
for quicker muscle education. The Neuro20 PRO System may also be used for
muscle recovery in the form of passively stimulating the muscles in a pattern that
is both relaxing and cleansing of metabolic waste signicantly deceasing the
effects of delayed onset muscle soreness (DOMS).
PRODUCT DESCRIPTION

10 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
Model Number: N20 PRO-SYS
The Neuro20 PRO System is designed to be operated for sessions for 1-10
users at a time. One Control Box is assigned per user and attaches to each
user’s Smart Suit. The operator controls the software for each user with individual
controls for each.

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 11
SYSTEM COMPONENTS AND OVERVIEW
To prevent any damage to the components we suggest carrying the Neuro20
PRO System in the provided Protective Case when traveling and during storage.
Protective Case for Neuro20 System (provided by 3rd party)
The Protective case is IP67 certied, water, dust and shock resistant and
provides protection for the technology. The Neuro20 PRO System is placed in
custom-cut foam to provide an additional layer of protection. The Smart Suit is
packaged separately.
Neuro20 PRO System Components:
• Neuro20 PRO Control Box - Model Number: N20PRO-CB
• Neuro20 PRO Software 1.0 - Model Number: N20PRO-SW
• Neuro20 Smart Suit 1.0 - Model Number: N20-SS
• Neuro20 PRO System Operating Manual: N20PRO-OM-V1.0
Neuro20 Protective Case
Neuro20 PRO System 3rd-Party Components:
• Operating Tablet w/charger
• Battery w/charger
• Protective case
Dispose of all batteries and component parts as per local regulations.
Do not dispose of batteries and the Operating Tablet in regular trash or
recycling bins unless local regulations permit.
Neuro20 Smart Suit Packaging

12 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
SYSTEM COMPONENTS AND OVERVIEW (continued)
Neuro20 PRO Control Box - Model Number: N20PRO-CB
The Neuro20 PRO Control Box is a powered muscle stimulator that attaches to
the Smart Suit. The Control Box generates electrical impulses and is controlled
by the operator through the Software installed on the Operating Tablet. The
Control Box wirelessly connects to operating tablet. The power supply of the
Control Box is provided through a rechargeable, replaceable battery.
Neuro20 PRO Control Box
Left LED
Blinking Red – Battery is too hot or battery is not connected properly.
Blinking Yellow – Remaining battery capacity is to low
Blinking Blue – the device is ready for connection
Blue – the device has an active wireless connection
Purple – the device is in rmware update mode
Blinking Purple – rmware update in progress
Blinking Green – device is performing initial self-test
Right LED
Green – the device is switched ON and in the idle state
Purple – Stimulation is ON
WhiteWhite – Rest Period of Stimulation
Both LED’s Blinking Red - failure of one or more stimulation components
2 RGB LEDs are located at the top of the cabinet and indicate the following states:

Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
www.neuro20.com | 13
SYSTEM COMPONENTS AND OVERVIEW (continued)
Battery & Charger
The battery & charger (LP-E5 battery, model LF7.4900) for the Neuro20 PRO
Control Box are provided by a 3rd party provider.
No other batteries other than model LP-E5 Li-ion should be used in the
Neuro20 PRO Control Box.
Typical operation time for a fully charged battery is 6 hours of use.
Typical shelf life for the battery is one year after full charge, and 500
charging cycles. Charging time for full charge is 3 hours.
USB/USB-C Charging cable
Battery
Contact Points
Battery & Charger
Dispose of all batteries and any equipment as per local regulations. Do
not dispose in regular trash or recycling bins unless local regulations
permit.
14 | www.neuro20.com
Neuro20 PRO System Operating Manual | © Neuro20™ | N20PRO-OM-V1.0 | 07/22
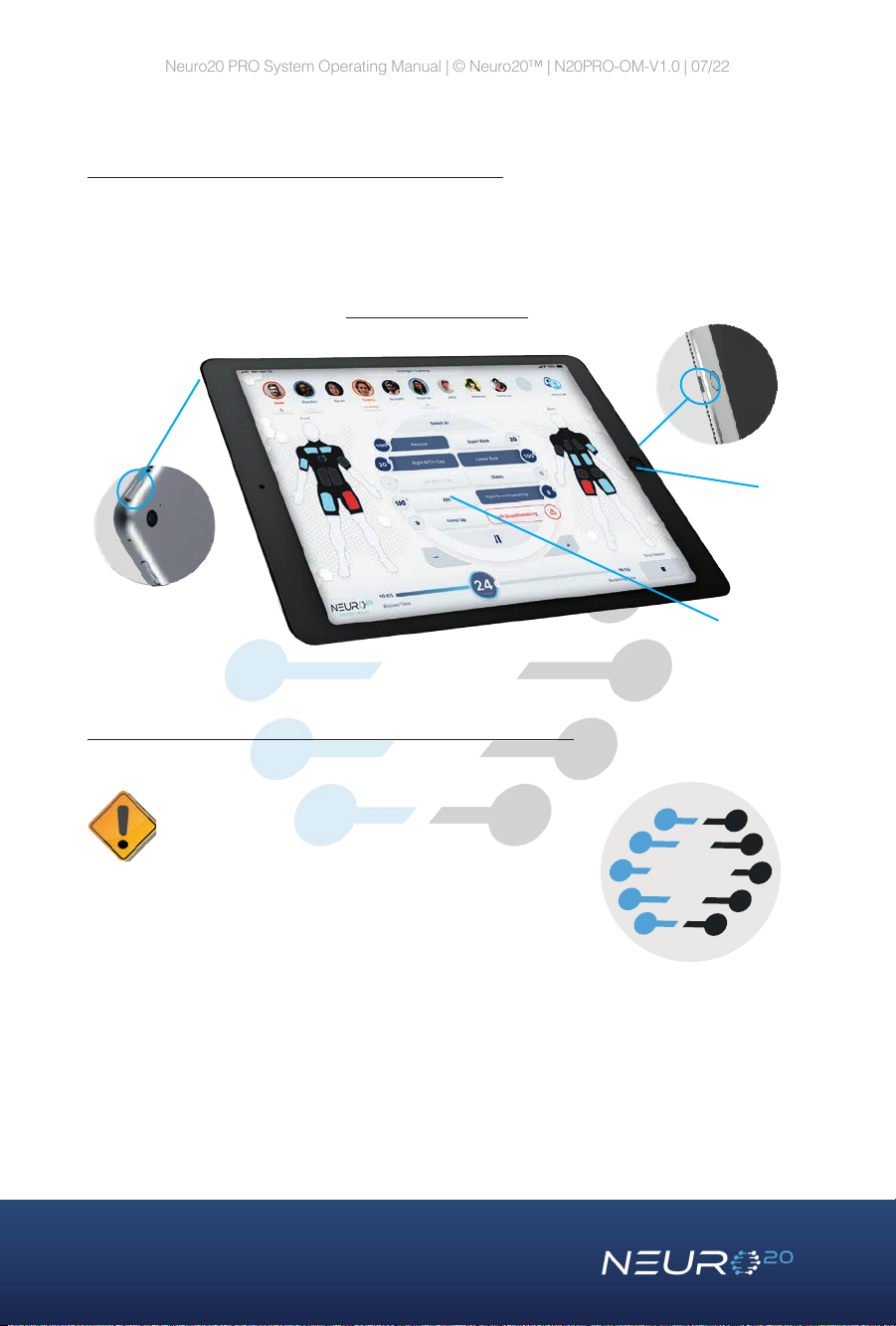
Operating Tablet – (Apple iPad, 9th generation)
The Operating Tablet comes with pre-installed Neuro20 PRO Software. The
tablet wirelessly connects to the Neuro20 PRO Control Box and operates the
Neuro20 PRO Software.
Neuro20 Operating Tablet
Home Button
Display Screen
Charging
Port
Power Button
SYSTEM COMPONENTS AND OVERVIEW (continued)
Neuro20 PRO Software - Model Number: N20PRO-SW
Software updates are issued on a regular
basis. Keep your Operating Tablet’s iOS
updated by ensuring that the App Store’s
automatic updates are ON. We suggest that
sole utilization of the provided Operating
Tablet is reserved for the Neuro20 PRO
Software.
Note- Neuro20 Technologies is not responsible for maintaining or installing any
software or application other than the Neuro20 PRO Software.
PRO
Table of contents
Popular Fitness Equipment manuals by other brands

G-FITNESS
G-FITNESS AIR ROWER user manual

CAPITAL SPORTS
CAPITAL SPORTS Dominate Edition 10028796 manual

Martin System
Martin System TT4FK user guide

CIRCLE FITNESS
CIRCLE FITNESS E7 owner's manual

G-FITNESS
G-FITNESS TZ-6017 user manual

Accelerated Care Plus
Accelerated Care Plus OMNISTIM FX2 CYCLE/WALK user manual











