Neuropace RNS 5000 Owner's manual

© 2018 NeuroPace, Inc.
DN 1017919 Rev 2
Rev Date: 2018-06
RNS®System Programming Manual
For the RNS®Tablet Model 5000 and the
Patient Data Management System (PDMS) Model 4340

ii
RNS®System Programming Manual
ABOUT THIS MANUAL
This manual includes instructions for use for:
•The RNS®Tablet model 5000 with software version 1.8
•The NeuroPace®Patient Data Management System model 4340
Note: The RNS®Tablet model 5000 is compatible with the RNS®Neurostimulator model
RNS-300M and model RNS-320.
Note: The term “programmer” as used in this manual is a generic term that refers to either the
RNS®Tablet or the NeuroPace®Programmer (laptop computer).
Note: Images in this manual are representative and may vary in detail from what a particular user
experiences.

iii
RNS®System Programming Manual
FCC INFORMATION
The following is communications regulation information on the neurostimulator models RNS-300M and
RNS-320,and wand model W-02.
•Neurostimulator model RNS-300M FCC ID: WBWRF300
•Neurostimulator model RNS-320 FCC ID: WBWRF320
•Wand FCC ID: WBW902
These components comply with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) These devices may not cause harmful interference, and (2) these devices must accept any
interference received, including interference that may cause undesired operation.
IMPORTANT: Changes or modifications to these components not expressly approved by NeuroPace, Inc.
could void the FCC Certification, and negate your authority to operate them.
This equipment complies with FCC radiation exposure limits set forth for an uncontrolled environment.
This transmitter must not be co-located or operating in conjunction with any other antenna or transmitter.

iv
RNS®System Programming Manual
SYMBOLS
Explanation of symbols on product or package labeling
Caution
Do Not Resterilize
MR Unsafe
Prescription Only
Non-Pyrogenic
Single Use
Sterilized Using Ethylene Oxide
Temperature Limits
Ethernet Connection (Network Connection)
Proposition 65, a State of California voter initiative, requires the following notice:
WARNING: This product canexpose you to chemicals including ethylene oxide, which is
known to the State of California to cause cancer and birth defects or other reproductive harm.
For more information go to www.P65Warnings.ca.gov.

1
RNS®System Programming Manual
TABLE OF CONTENTS
ABOUT THIS MANUAL......................................................................................................II
FCC INFORMATION ........................................................................................................III
SYMBOLS..................................................................................................................... IV
INTRODUCTION.............................................................................................................4
DEVICE DESCRIPTION.....................................................................................................4
RNS®TABLET...............................................................................................................4
NEUROPACE®PATIENT DATA MANAGEMENT SYSTEM ......................................................4
PRODUCTS AND ACCESSORIES .......................................................................................4
CONTACTING NEUROPACE .............................................................................................5
TYPOGRAPHIC CONVENTIONS .........................................................................................5
WARNINGS AND CAUTIONS –PROGRAMMER....................................................................6
STERILIZATION AND STORAGE ........................................................................................8
Product Storage....................................................................................................................................8
Explant and Disposal............................................................................................................................8
Wand Cleaning and Sterilization ..........................................................................................................8
Electrostatic Discharge (ESD) / Static Electricity.................................................................................9
RNS®TABLET INSTRUCTIONS..................................................................................10
POWER ON THE TABLET...............................................................................................10
CONNECT AND LOGIN OVERVIEW ..................................................................................10
FUNCTIONALITY WITH AND WITHOUT A PDMS CONNECTION ............................................11
CONNECT TO THE INTERNET FOR PDMS ACCESS...........................................................11
Mobile Broadband Works Automatically.............................................................................................11
Steps to Connect to a Wi-Fi Network.................................................................................................12
If You Lose Network Connectivity ......................................................................................................13
LOG IN TO THE RNS®TABLET ......................................................................................14
LOG IN TO THE PDMS..................................................................................................15
INTERROGATE THE RNS®NEUROSTIMULATOR ...............................................................16
DATA RETENTION AND ACCESS ON THE NEUROSTIMULATOR,PROGRAMMER AND PDMS .17
Programmed Settings.........................................................................................................................17
Storage of ECoGs and Neurostimulator Activity (Diagnostics)..........................................................17
HOME SCREEN ............................................................................................................18
NEUROSTIM INFO SCREEN ............................................................................................20
Electrode Impedance and Battery Voltage.........................................................................................22
ACTIVITY SCREEN........................................................................................................23
Activity Histogram Viewing Options....................................................................................................24
View Neurostim Settings ....................................................................................................................25
View Event List...................................................................................................................................28
Types of Reports................................................................................................................................28

2
RNS®System Programming Manual
Advanced Views.................................................................................................................................29
ECOGLIBRARY SCREEN..............................................................................................29
LIVE ECOGS SCREEN..................................................................................................31
CREATE THE RECORDING MONTAGE .............................................................................32
Identifying Ports, Leads and Electrodes on the Neurostimulator.......................................................33
Creating the Montage.........................................................................................................................34
Recommended Initial Montage Settings.............................................................................................35
Program the Neurostimulator with the New Settings .........................................................................37
SET UP ECOGCAPTURE..............................................................................................38
Selecting ECoG Capture Settings......................................................................................................38
Recommended Initial ECoG Capture Settings...................................................................................41
Program the Neurostimulator with the New Settings .........................................................................43
RECOMMENDATION FOR REVIEW OF INITIAL ECOGCAPTURE SETTINGS ..........................43
CONFIGURE PATTERN DETECTION:DEFINE AND MODIFY DETECTION SETTINGS ...............44
Phase 1: Select a Default Detection Set upon First Use....................................................................45
Phase 2: Define a Pattern Detection Set ...........................................................................................48
Phase 3: Customize Pattern Detection ..............................................................................................52
DETECTION TOOLS AND DETECTORS.............................................................................56
Detection Tools and Detectors...........................................................................................................56
Power Change Detector.....................................................................................................................57
Rhythmic Activity Detector .................................................................................................................59
Spike Activity Detector .......................................................................................................................60
Bandpass Detection Tool ...................................................................................................................61
Area Detection Tool............................................................................................................................62
Advanced Controls.............................................................................................................................63
SHARE AND/OR DOWNLOAD A DETECTION SET ..............................................................64
Steps to Share a Detection Set..........................................................................................................64
Steps to Download a Detection Set to the Tablet ..............................................................................66
OVERVIEW OF RESPONSIVE THERAPY ...........................................................................69
CONFIGURE RESPONSIVE THERAPY ..............................................................................70
Optional Advanced Settings...............................................................................................................74
Test Stimulation Before Enabling.......................................................................................................77
Enable Therapy: Review & Program..................................................................................................78
Recommended Initial Responsive Therapy Settings .........................................................................79
Recommended Therapy Modifications After Observing Clinical Response.......................................80
EXPORT AND IMPORT DEVICE SETTINGS FOR NEUROSTIMULATOR REPLACEMENT ............81
Transfer Compatibility.........................................................................................................................81
Export Neurostimulator Settings.........................................................................................................81
Import Neurostimulator Settings.........................................................................................................82
PATIENT LIST ON TABLET AND THE PDMS ON A PERSONAL COMPUTER ........84
PDMS ACCESS ON A PERSONAL COMPUTER.................................................................85
PDMS PROFILE AND SETTINGS ....................................................................................85
PATIENT &NEUROSTIM INFO ........................................................................................88
NEUROSTIM SETTINGS..................................................................................................88

3
RNS®System Programming Manual
ACTIVITY.....................................................................................................................90
ECOGLIBRARY...........................................................................................................90
LIVE ECOGS...............................................................................................................90
PATIENT FOLLOW-UP ACTIVITIES............................................................................91
TROUBLESHOOTING..................................................................................................92
Network Connectivity Problems .........................................................................................................92
Abnormal Lead Impedance (greater than 3500 Ohms or less than 250 Ohms)................................92
Insufficient Charge..............................................................................................................................95
Noise, Artifacts, Poor Signal Displayed, or No Signal Displayed in Live ECoG.................................95
Poor or No Communication Between the RNS®Neurostimulator and the RNS®Tablet ...................96
Tablet Does Not Turn On ...................................................................................................................97
Tablet Freezes or Does Not Turn Off.................................................................................................97
Tablet Shuts Down.............................................................................................................................97
Impedance Measurement Was Rejected / Test Request Was Rejected...........................................97
SPECIFICATIONS AND CHARACTERISTICS ............................................................98
RNS®TABLET.............................................................................................................98
TABLET WIRELESS.......................................................................................................98
RNS®SYSTEM WIRELESS............................................................................................99
ELECTROMAGNETIC EMISSIONS AND IMMUNITY ..............................................................99
Hospital or Medical Environments......................................................................................................99
Home, Work or Public Environments ...............................................................................................100
Guidance and Manufacturer’s Declaration.......................................................................................100
Emissions and Immunity Information ...............................................................................................101
GLOSSARY................................................................................................................105
INDEX.........................................................................................................................110

Introduction 4
RNS®System Programming Manual
INTRODUCTION
DEVICE DESCRIPTION
•RNS®Tablet model 5000 with software version 1.8
•NeuroPace®Patient Data Management System model 4340
RNS®TABLET
The RNS®Tablet includes a tablet computer that runs proprietary NeuroPace software, and uses custom
telemetry components to communicate with the RNS®Neurostimulator. Clinicians can use the tablet to
program how the neurostimulator operates. Settings include, but are not limited to, the implanted system
configuration, detection settings adapted to the patient's ECoG patterns,settings for ECoG record
storage, and settings of the therapies. Clinicians can also use the tablet to review previously retrieved
neurostimulator activity information, perform detection analysis, and communicate with the NeuroPace®
Patient Data Management System (PDMS) via the Internet.
Note: The term “programmer” as used in this manual is a generic term that refers to either the
RNS®Tablet or the NeuroPace®Programmer (laptop computer).
NEUROPACE®PATIENT DATA MANAGEMENT SYSTEM
The Patient Data Management System (PDMS) maintains patient and product data obtained from the
programmer and remote monitor. Authorized users may access the PDMS via the Internet using a
personal computer. An electronic signature in the form of a username and password are required for user
authentication.
PRODUCTS AND ACCESSORIES
Note: Manuals and other literature are not listed below.
RNS
®
Tablet Kit
Model 5001
Item
Model Number
RNS®Tablet (with attached case)
5000
Stylus
AC Adapter
Carrying Bag
Used With
Wand
W-02

Introduction 5
RNS®System Programming Manual
This manual contains information applicable to the RNS®Tablet, which is used to program how
the RNS®Neurostimulator operates. It includes information about the secure Patient Data
Management System (PDMS) database, which is accessible using the tablet or a web browser
on a personal computer.
Refer to the RNS®System manual for additional device descriptions, device specifications, and
indications for use, contraindications, warnings, cautions, and instructions for use.
All NeuroPace®manuals are available at www.NeuroPace.com or by contacting NeuroPace,
Inc. (see Contacting NeuroPace on page 4).
Refer to the clinical summary booklet for information on the clinical study results of the RNS®
System and adverse event data.
CONTACTING NEUROPACE
All questions or concerns regarding the NeuroPace®RNS®System should be forwarded to:
NeuroPace, Inc.
455 N. Bernardo Ave.
Mountain View, CA 94043
Customer Support: 866-726-3876 (Toll Free in the United States)
Website: www.neuropace.com
TYPOGRAPHIC CONVENTIONS
This manual uses the following typographic conventions.
WARNING: WARNING TITLE
Warnings alert you to serious adverse events and potential safety hazards and
situations that may cause injury.
Caution: Caution Title
Cautions alert you to exercise special care.
Note: Notes provide additional information.
1. Numbered lists are used to identify a sequence of steps.
This format is used to identify figure titles and descriptions
BOLD SMALL CAPS indicate on-screen text, like the names of screens, buttons and fields.
Note: Software version numbers found on screenshots in this manual are provided for illustrative
purposes only and may not be the same as the version number you see on screen.

Introduction 6
RNS®System Programming Manual
WARNINGS AND CAUTIONS –PROGRAMMER
Note: See the RNS®System manual for warnings and cautions regarding other components of
the RNS®System.
WARNING: PHYSICIAN AND CENTER ACCESS TO THE RNS®SYSTEM
The RNS®System should only be implanted by neurosurgeons with adequate
experience in the implantation of subdural and stereotactic implantation of
intraparenchymal electrodes and in the surgical treatment of intractable epilepsy. The
RNS®System should only be used by neurologists or neurosurgeons with adequate
experience in the management of intractable epilepsy and in the localization of
epileptic foci, including the use of scalp and intracranial electrodes.
Neurologists and neurosurgeons using the RNS®System must have completed the
NeuroPace®RNS®System training program. To qualify to manage patients with the
RNS®System, physicians must demonstrate specific expertise related to epilepsy,
video-EEG monitoring, interpretation of electrocorticograms (ECoGs), the
pharmacology of antiepileptic medications and selection of patients for epilepsy
surgery. Implantation of the RNS®System should be performed only by qualified
neurosurgeons at centers capable of providing comprehensive epilepsy care, i.e.
“Comprehensive Epilepsy Centers.” These centers should have the expertise to
provide diagnostic services that include video-EEG monitoring with scalp and
intracranial electrodes and neuroimaging, and are experts in the treatment of epilepsy
with antiepileptic medications, epilepsy surgery, and devices.
WARNING: MANAGEMENT OF PATIENTS WITH THE RNS®SYSTEM BY PHYSICIANS AT CENTERS THAT DO
NOT PROVIDE THE SERVICES PROVIDED AT COMPREHENSIVE EPILEPSY CENTERS
In some instances, post-implant programming may be conducted by neurologists
meeting the experience and certification requirements for neurologists at
Comprehensive Epilepsy Centers, but who are not practicing in such centers. This
situation might occur if the patient is not able to travel to a Comprehensive Epilepsy
Center for regular follow-up (e.g. because of distance from the Center or limited
access to transportation). These neurologists will be qualified by NeuroPace to provide
post-implant programming. After NeuroPace®RNS®System training is complete, the
qualified programming neurologist may receive external NeuroPace products
(programmer, remote monitor).
WARNING: POTENTIAL SHOCK
Submerging any part of the programmer, or operating the programmer in or near a wet
environment, may result in an electrical shock.
The programmer must be disconnected from the electrical outlet prior to cleaning to
avoid the potential of electrical shock.
Electrical shock may occur if the programmer AC adapter and power cord are not
properly connected to a grounded power source.
WARNING: RADIO FREQUENCY IDENTIFICATION (RFID) INTERFERENCE
Sources of RFID can result in signals that appear as ECoG activity to the
neurostimulator. Signals that appear as ECoG activity could also result in delivering

Introduction 7
RNS®System Programming Manual
the programmed stimulation to the patient (per the device detection programming).
The physician should be aware of possible sensing artifacts when assessing the
ECoG recordings. Potential sources of RFID may occur in a health care environment,
retail stores, public libraries, airports and business environments.
Refer to Electromagnetic Emissions and Immunity on page 99 for more
information.
WARNING: SECURITY AND ELECTRONIC TRACKING SYSTEMS
Security screening devices (such as theft detectors, security tag deactivators, and
airport security screening devices) can result in signals that appear as ECoG activity to
the neurostimulator. Signals that appear as ECoG activity could also result in
delivering the programmed stimulation to the patient (per the device detection
programming). Such devices may be found at retail stores, public libraries and
airports. The physician should be aware of possible sensing artifacts when assessing
the ECoG recordings. Patients should be instructed to walk through the center of such
security screening units without stopping, when possible, and exit the area of the
screening device as soon as possible.
WARNING: NEUROPACE COMPONENTS
Use of accessories, transducers, and cables other than those provided by NeuroPace
could result in increased electromagnetic emissions or decreased electromagnetic
immunity of the RNS®System and result in improper operation.
WARNING: PORTABLE AND MOBILE RADIO FREQUENCY (RF) COMMUNICATIONS EQUIPMENT
Portable and mobile RF communications equipment (including peripherals such as
antenna cables and external antennas) should be used no closer than 12 inches (30
cm) to any part of the RNS®System, including cables. Otherwise, degradation of the
performance of the RNS®System could result.
WARNING: NEUROPACE®EQUIPMENT PLACEMENT
Use of NeuroPace®equipment (for example, remote monitor or programmer) adjacent
to or stacked with other equipment should be avoided because it could result in
improper operation. If such use is necessary, the NeuroPace®equipment and other
equipment should be observed to verify that they are operating normally.
Caution: Telemetry Artifact
Telemetry may produce an electrographic artifact. If responsive therapy is enabled
with a sensitive detection set, detection of the electrographic artifact may occur
resulting in therapy delivery. The physician should be aware of possible sensing
artifacts when assessing the ECoG recordings.
Caution: Afterdischarge Activity
If evidence of afterdischarge activity resulting from stimulation is seen either on stored
ECoGs or during test stimulation delivery, stimulation parameters should be adjusted
to prevent such occurrence.

Introduction 8
RNS®System Programming Manual
Caution: Programmer Failure
As with any electronic device, the programmer may be damaged or malfunction if the
programmer AC adapter and power cord are not properly connected to a grounded
power source.
Caution: Incompatibility of Programmer with Other Medical Devices
The effects of using the programmer to interrogate other electronic, programmable
devices such as pacemakers, defibrillators, cochlear implants, and other
neurostimulators or CPAP machines are unknown. It could result in reprogramming of
the other device and therefore, the physicians familiar with each device should check
the programmed parameters of each device before the patient is discharged and after
each programming session of either device.
Caution: Electronic Interference
Communications between the programmer and the implanted neurostimulator may be
interrupted by emissions from nearby electronic devices. Examples of sources of EMI
are lithotripsy, computer monitors, cellular telephones, motorized wheel chairs, x-ray
equipment and other monitoring equipment. Interruption of telemetry can result in
incomplete communication. If EMI disrupts programming, move the programmer away
from the likely source of EMI. Refer to Poor or No Communication Between the
RNS®Neurostimulator and the RNS®Tablet (page 96) for more information.
Caution: Placement of the Programmer Power Cord
Make sure nothing rests on the programmer power cord and that the cord is not
located where it can be tripped over or stepped on.
Caution: Heating
The programmer’s AC adapter may become hot during normal operation. Use care
when handling during or immediately after operation.
STERILIZATION AND STORAGE
Product Storage
Components should be stored in a clean and secure area with a room temperature of approximately
14 to 28 degrees Celsius.
Explant and Disposal
Program all detection and therapy functions to DISABLED prior to explanting and shipping the RNS®
Neurostimulator. Return the explanted neurostimulator and leads to NeuroPace. NeuroPace will
provide shipping containers if requested.
DO NOT incinerate the neurostimulator; explosion can occur if the neurostimulator is exposed to
incineration or cremation temperatures.
Wand Cleaning and Sterilization
The wand can be cleaned by wiping with water. It can be placed in a sterile bag for use in the sterile
field. DO NOT STERILIZE the wand.

Introduction 9
RNS®System Programming Manual
Electrostatic Discharge (ESD) / Static Electricity
The ports on the tablet may be sensitive to electrostatic discharge / static electricity. Handle the tablet
ports carefully. If exposed to electrostatic discharge, the tablet may experience telemetry artifacts or
errors, or may freeze. In the event of a tablet freeze, refer to Tablet Freezes
on page 97.

Tablet Instructions 10
RNS®System Programming Manual
RNS®TABLET INSTRUCTIONS
This chapter primarily addresses use of the tablet during an interactive programming session, that is,
when the clinician uses the wand for communication between the neurostimulator and tablet. For
information on using the PDMS apart from an interactive programming session, either using the tablet or
using a web browser on a personal computer, see Patient List on Tablet and the PDMS on a Personal
Computer on page 84.
POWER ON THE TABLET
When interacting with a patient, the tablet should be used on battery power only. Make sure the tablet is
sufficiently charged for use before powering on.
Press and hold the power button on the upper right side of the tablet. After 2-3 seconds, the tablet beeps
to indicate it is powering on.
CONNECT AND LOGIN OVERVIEW
Access to all of the functionality of the tablet requires an Internet connection and two logins, in this order:
1. Connect to the Internet via Wi-Fi or mobile broadband on the tablet. Mobile broadband works
automatically. To connect to Wi-Fi, see Steps to Connect to a Wi-Fi Network on page 12.
Alternatively, the tablet computer can also support use of a docking station that has an Ethernet
connector (wired connection). Contact NeuroPace customer service if you prefer this alternative.
2. Login to the tablet.NeuroPace provides the initial username and password for this login.
3. Login to the Patient Data Management System (PDMS),which requires the Internet
connection established in the first step. NeuroPace also provides the initial username and
password for the PDMS. (For PDMS access using a personal computer, select the PDMS link at
www.neuropace.com and log in. For further instructions, see PDMS Access on a Personal
Computer on page 85.)
Note: Programming functions that are typically performed in the operating room do not require an
Internet connection to the PDMS.
You can access all programming functions without connection to the PDMS except the following:
•View stored information such as ECoGs, neurostimulator activity and settings for any patient
•Make a new detection set or adjust parameters of a detection set
For details, see Functionality with and without a PDMS Connection on page 11.

Tablet Instructions 11
RNS®System Programming Manual
FUNCTIONALITY WITH AND WITHOUT A PDMS CONNECTION
Note: Programming functions that are typically performed in the operating room do not require an
Internet connection to the PDMS.
The table below identifies the tablet functions that do not require an Internet connection to the PDMS and
those that do.
Function Internet Connection to
the PDMS Required
Interrogate the neurostimulator No
View neurostimulator information and settings downloaded during current
session, including battery voltage, impedance, stimulation settings,
recording montage
No
Create or change the recording montage No
Set up or adjust ECoG capture settings No
Select and program a default detection set or a custom detection set
previously saved to the tablet from the PDMS No
Select and program all stimulation settings No
View stored information such as ECoGs, neurostimulator activity and
settings for any patient Yes
Make a new detection set or adjust parameters of a detection set Yes
CONNECT TO THE INTERNET FOR PDMS ACCESS
When connected to the Internet and logged in to the Patient Data Management System (PDMS), the
RNS®Tablet provides a look-through to the information stored on the PDMS, such as ECoGs,
neurostimulator settings and activity. The PDMS is a patient database integrated with the RNS®Tablet;
the PDMS is also available using a browser on any Internet-connected computer. The tablet ships with
Wi-Fi enabled, so it can detect available Wi-Fi networks out of the box.
Note: If you attempt to connect to a Wi-Fi network that requires you to go to an Internet website to
log in, as in some hotels and coffee shops, you will not be able to log in because the tablet does
not have an Internet browser program. As a medical device, its Internet connectivity is restricted
to the PDMS only.
The tablet ships with Wi-Fi and mobile broadband enabled.
Mobile Broadband Works Automatically
Mobile broadband is pre-configured by NeuroPace to be on and working, so no further effort is
necessary and the tablet will connect to mobile broadband automatically if a signal is available.
Tablet Instructions 12
RNS®System Programming Manual
Steps to Connect to a Wi-Fi Network
Follow these steps to connect via Wi-Fi to the Internet on the RNS®Tablet.
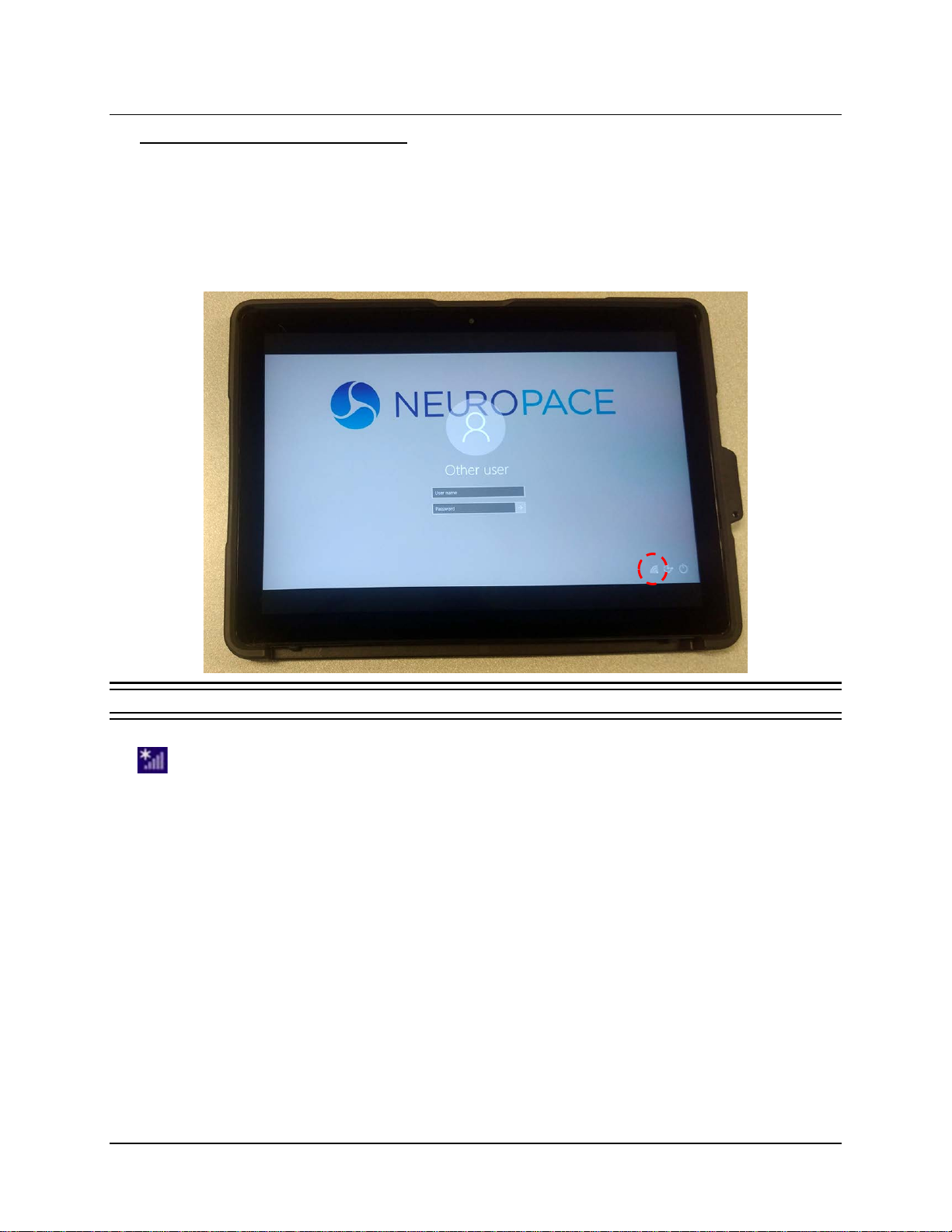
1. The tablet login screen appears first upon startup. Select a user. The screen prompts you for
a username and password and displays the keyboard, but ignore these for now.
2. Touch to select the small symbol that indicates network status. (If necessary, touch outside
the login fields to dismiss the keyboard and see the whole screen.)
Figure 1 Touching the network icon at lower left
When a wireless network is available, it looks like the signal strength symbol with an asterisk
above it. A different symbol is present if there is no wireless network available. Select it in any
case to open the NETWORKS panel on the right side of the screen.

Tablet Instructions 13
RNS®System Programming Manual
3. From this point, Wi-Fi access is the same as on a smartphone or laptop computer:
a. Select the desired network and then select CONNECT.
b. Enter the network security key and select NEXT.(The security key is your Wi-Fi
password.) The tablet connects if the security key is correct. Internet connection is
complete.
If You Lose Network Connectivity
If you lose network connectivity, you will not lose information you already programmed into the
neurostimulator or saved to the PDMS, but you will lose information not already programmed into the
neurostimulator or saved to the PDMS.
If you are already logged in to the tablet and have lost network connectivity, first log off to return to
the login screen: return to the Home screen and touch the circle-X (exit) button at upper right, and
then select EXIT on the PROGRAMMER EXIT dialog. Then follow the Steps to Connect to a Wi-Fi
Network on page 12. If you still cannot connect, contact NeuroPace for further assistance (see
Contacting NeuroPace on page 4).

Tablet Instructions 14
RNS®System Programming Manual
LOG IN TO THE RNS®TABLET
This is the first of two logins. The second one gives you access to the PDMS.
1. The tablet login screen appears first upon startup. Select a user. The screen prompts you for a
username and password and displays the keyboard.
2. Enter your username and password (initially provided by NeuroPace) and select Enter. Login
completes if the password is correct and then the NEUROPACE LOG INscreen appears, which lets
you login to the PDMS, as described next.
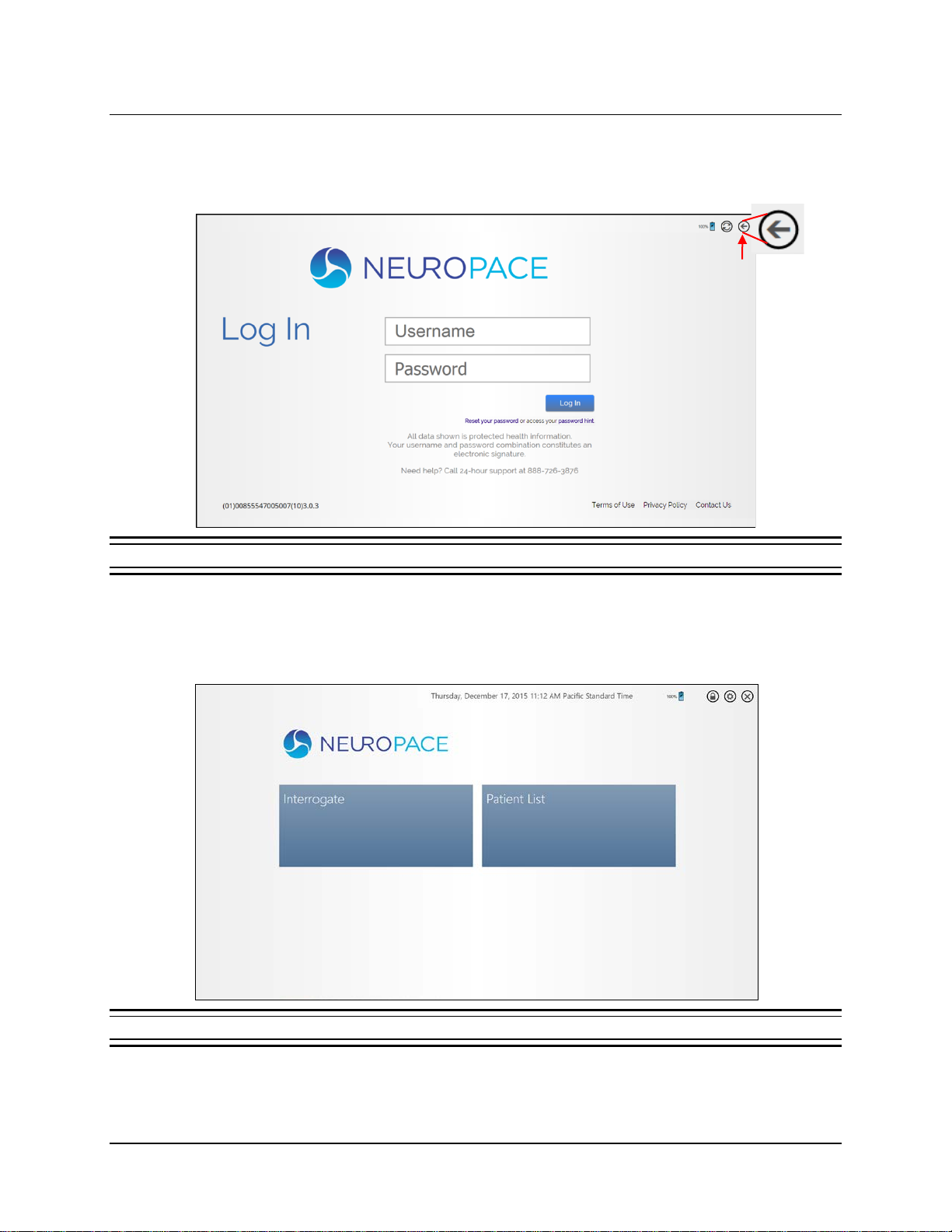
Tablet Instructions 15
RNS®System Programming Manual
LOG IN TO THE PDMS
The NEUROPACE LOG INscreen appears after tablet login, and whenever you attempt to access functions
that require PDMS access, like the PATIENT LIST and ECOGLIBRARY.
Figure 2 NeuroPace Log In screen—for PDMS login
To skip PDMS login, select the BACK button in the upper right corner. The Start screen opens.
1. Select the fields to enter the username and password and then select LOG IN. Login completes
and the Start screen opens (Figure 3).
Figure 3 Start screen
If Internet not available, select Back
to go to Home screen without
logging into the PDMS.
Tablet Instructions 16
RNS®System Programming Manual
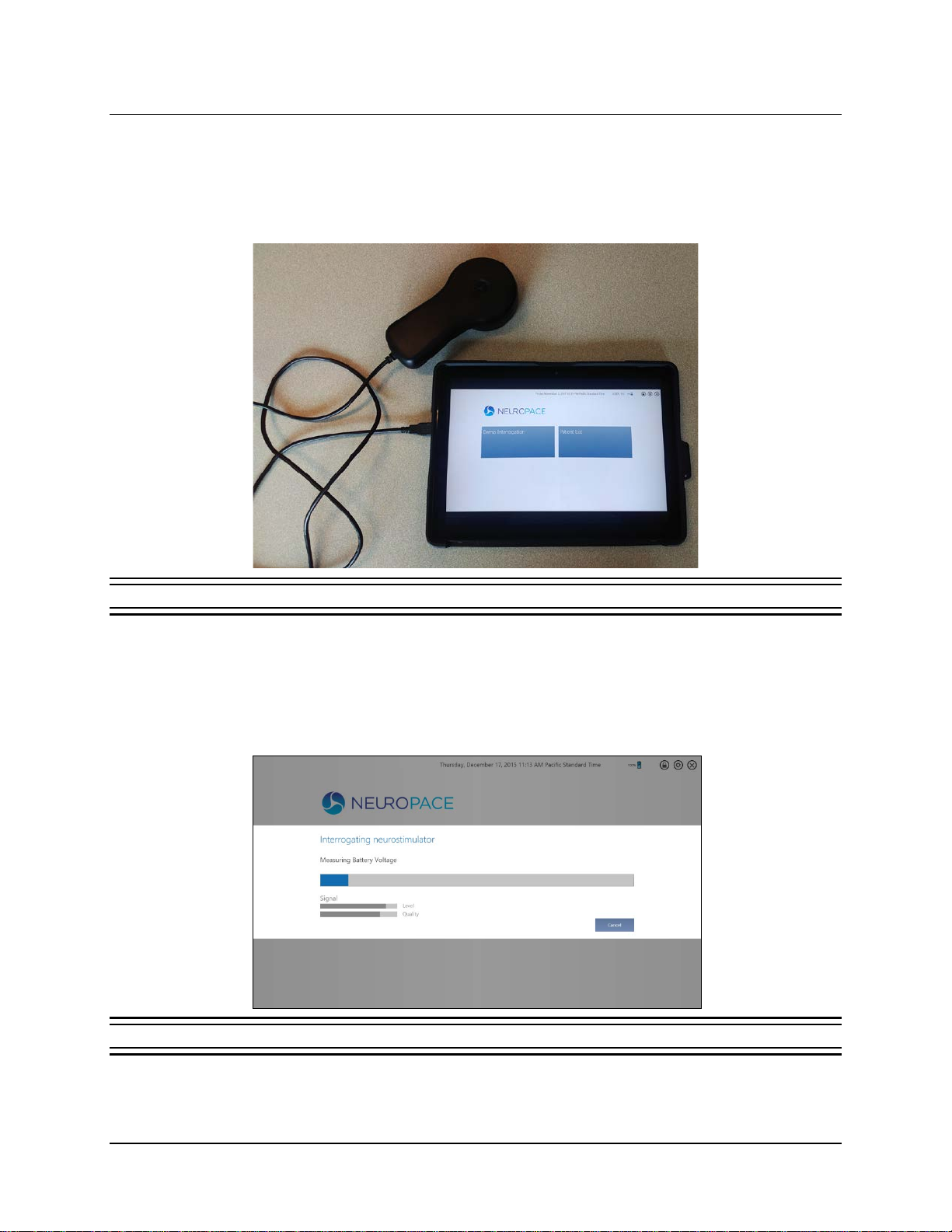
INTERROGATE THE RNS®NEUROSTIMULATOR
To interrogate the RNS®Neurostimulator means to identify it, perform routine tests and measurements,
and retrieve currently programmed settings and stored data from it. Follow these steps to interrogate:
1. Connect the wand to the USB port on the tablet.
Figure 4 Tablet with connected wand
2. Place the wand within approximately 1 inch of the neurostimulator, concave side of wand
facing the neurostimulator. Then select INTERROGATE from the Start screen (left tile—see
Figure 3). You can also select the green INTERROGATE tile at upper right in the HOME screen
(see Figure 6.) The wand must be held in place over the neurostimulator for interrogation to
succeed.
Figure 5 Interrogating neurostimulator dialog—interrogation in progress
This manual suits for next models
1
Table of contents
Other Neuropace Medical Equipment manuals
Popular Medical Equipment manuals by other brands

human care
human care Ready Stand manual

Olympus
Olympus EVIS EUS EU-ME2 Quick reference guide

Biegler
Biegler autopress Instructions for use

Sunrise Medical
Sunrise Medical JAY Series Installation sheet

SYAS Technology
SYAS Technology PREMIUM BED 4 MOTOR Manual book
Defibtech
Defibtech DDU-2000 Series operating guide