Neuropace RNS System User manual

Rev Date: 2019-01 DN 1017309 Rev 4
RNS®System User Manual
© 2019 NeuroPace, Inc.

ii
This Manual supports:
•RNS®Neurostimulator: model RNS-300M with firmware version 7.0
•NeuroPace®Cortical Strip Lead: models CL-315-10, CL-325-10, CL-335-10
•NeuroPace®Depth Lead: models DL-330-3.5, DL-330-10, DL-344-3.5,
DL-344-10
•NeuroPace®Programmer: model PGM-300 with software version 1.6
FCC Information
The following is communications regulation information on the model RNS-300M
neurostimulator and model W-02 wand.
Neurostimulator FCC ID: WBWRF300
Wand FCC ID: WBW5200 or WBW902
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2)
this device must accept any interference received, including interference that may
cause undesired operation.
IMPORTANT: Changes or modifications to this product not expressly approved by
NeuroPace, Inc. could void the FCC Certification, and negate your authority to operate
them.
This equipment complies with FCC radiation exposure limits set forth for an uncontrolled
environment. This transmitter must not be co-located or operating in conjunction with
any other antenna or transmitter.

Table of Contents 1
RNS®System User Manual
TABLE OF CONTENTS
INTRODUCTION........................................................................................................................................... 5
CONTACTING NEUROPACE .......................................................................................................................... 5
ABOUT THIS MANUAL................................................................................................................................... 5
RNS®SYSTEM ............................................................................................................................................. 8
INDICATIONS ............................................................................................................................................... 8
CONTRAINDICATIONS................................................................................................................................... 8
WARNINGS AND PRECAUTIONS .................................................................................................................... 8
IMPLANTED RNS®SYSTEM........................................................................................................................ 18
RNS®SYSTEM DESCRIPTION .................................................................................................................... 18
RNS®NEUROSTIMULATOR ........................................................................................................................ 21
RNS®SYSTEM COMPONENTS AND ACCESSORIES ...................................................................................... 21
NEUROPACE®CORTICAL STRIP LEAD ........................................................................................................ 22
NEUROPACE®DEPTH LEAD ....................................................................................................................... 22
LEAD COMPONENT AND ACCESSORIES....................................................................................................... 23
NEUROPACE®PROGRAMMER .................................................................................................................... 23
TELEMETRY WAND .................................................................................................................................... 24
NEUROPACE®PATIENT DATA MANAGEMENT SYSTEM ................................................................................. 24
STERILIZATION,STORAGE,AND HANDLING ................................................................................................. 25
RNS®SYSTEM LONGEVITY........................................................................................................................ 26
PRODUCT REGISTRATION .......................................................................................................................... 28
CLINICAL USE OF THE RNS®SYSTEM .................................................................................................. 29
IDENTIFYING CANDIDATES FOR THE RNS®SYSTEM THERAPY...................................................................... 29
LOCALIZING THE SEIZURE FOCUS AND PLANNING LEAD LOCATION .............................................................. 29
PATIENT TRAINING .................................................................................................................................... 30
OVERVIEW OF IMPLANTATION AND PROGRAMMING OF THE RNS®SYSTEM ................................................... 30
Implanting the RNS®Neurostimulator and Leads .............................................................................. 30
Recommended Initial Detection and ECoG Storage Settings............................................................ 30
Recommended Initial Responsive Therapy Settings ......................................................................... 31
Modifying Detection and Responsive Therapy Settings..................................................................... 32
SURGICAL PROCEDURES ....................................................................................................................... 33
PRE-IMPLANT............................................................................................................................................ 33
RECOMMENDED IMPLANT PROCEDURE FLOW CHART .................................................................................. 35
RECOMMENDED NEUROPACE®DEPTH LEAD IMPLANTATION AND FIXATION .................................................. 36
RECOMMENDED NEUROPACE®CORTICAL STRIP LEAD IMPLANTATION AND FIXATION.................................... 39
RECOMMENDED RNS®NEUROSTIMULATOR IMPLANTATION PROCEDURE ..................................................... 40
CONNECTING THE RNS®NEUROSTIMULATOR TO THE IMPLANTED LEAD(S)................................................... 44
REPLACING /EXPLANTING THE RNS®SYSTEM ........................................................................................... 48
CHANGING THE LEADS THAT ARE CONNECTED TO THE NEUROSTIMULATOR .................................................. 51
PROGRAMMING INSTRUCTIONS............................................................................................................ 54
LOGGING ONTO THE NEUROPACE®PROGRAMMER...................................................................................... 54
TESTING THE WAND SIGNAL ...................................................................................................................... 54
INTERROGATING THE RNS®NEUROSTIMULATOR ........................................................................................ 54
IMPEDANCE MEASUREMENTS..................................................................................................................... 55
BATTERY VOLTAGE MEASUREMENTS ......................................................................................................... 55
CAPTURING REAL-TIME ECOGS................................................................................................................. 55
OBTAINING NEUROSTIMULATOR ACTIVITY INFORMATION ............................................................................. 56
REVIEWING ECOGRECORDS RETRIEVED FROM THE RNS®NEUROSTIMULATOR.......................................... 56
DELIVERING TEST STIMULATIONS............................................................................................................... 57
SYNCHRONIZING THE PROGRAMMER WITH THE NEUROPACE®PATIENT DATA MANAGEMENT SYSTEM ........... 58
PATIENT INFORMATION AND PHYSICIAN EMERGENCY CONTACT INFORMATION.............................................. 59

Table of Contents 2
RNS®System User Manual
Entering Patient Information into the Programmer............................................................................. 59
Entering Physician Emergency Contact Information.......................................................................... 59
Programming Information ................................................................................................................... 59
ASSIGNING LEAD LABELS AND CREATING THE MONTAGE............................................................................. 59
Assigning the Lead Labels ................................................................................................................. 59
Recommended Initial Lead Labels ..................................................................................................... 61
Creating a Montage ............................................................................................................................ 62
Recommended Initial Montage settings ............................................................................................. 62
Programming the Newly selected Settings ........................................................................................ 63
SETTING UP ECOGSTORAGE.................................................................................................................... 63
Selecting ECoG Storage Settings ...................................................................................................... 63
Recommended Initial ECoG Storage Settings ................................................................................... 64
Recommended Modifications to ECoG Storage Settings .................................................................. 66
Programming the Newly Selected ECoGs Storage Settings.............................................................. 67
SETTING UP DETECTION ............................................................................................................................ 68
Enabling, Disabling, or Changing the Detection Settings .................................................................. 68
Recommended Initial Detection Settings ........................................................................................... 69
Recommended Modifications to Detection Settings........................................................................... 70
Programming the Newly Selected Detection Settings........................................................................ 74
SETTING UP RESPONSIVE THERAPY ........................................................................................................... 75
Enabling, Disabling, or Changing Responsive Therapy..................................................................... 76
Recommended Initial Responsive Therapy Settings ......................................................................... 77
Recommended Modifications to Responsive Therapy Settings......................................................... 80
Programming the Newly Selected Settings ........................................................................................ 80
REVIEWING REPORTS................................................................................................................................ 81
MAGNET..................................................................................................................................................... 82
PATIENT FOLLOW-UP ACTIVITIES ......................................................................................................... 83
TROUBLESHOOTING................................................................................................................................ 84
DAMAGED PRODUCTS ............................................................................................................................... 84
GENERAL TROUBLESHOOTING ACTIVITIES .................................................................................................. 84
Abnormal Lead Impedance (greater than 3500 Ohms or less than 250 Ohms) ................................ 84
Insufficient Charge.............................................................................................................................. 86
Noise, Artifacts, Poor Signal Displayed, or No Signal Displayed in Real-time ECoG........................ 86
Poor or No Communication Between the RNS®Neurostimulator and the Programmer.................... 87
Programmer Turns OFF or Freezes................................................................................................... 88
Low Battery Measurement ................................................................................................................. 88
Impedance Measurement was Rejected / Test Request was Rejected............................................. 88
RNS®Neurostimulator Reset (DC Leak Detected) ............................................................................ 88
SPECIFICATIONS AND CHARACTERISTICS.......................................................................................... 90
RNS®NEUROSTIMULATOR ........................................................................................................................ 90
RNS®SYSTEM WIRELESS ......................................................................................................................... 91
RNS®SYSTEM LEADS............................................................................................................................... 91
IMPLANTABLE RNS®SYSTEM COMPONENT AND ACCESSORY MATERIALS .................................................... 92
NEUROPACE®PROGRAMMER .................................................................................................................... 92
WAND (MODEL W-02)............................................................................................................................... 92
ELECTROMAGNETIC EMISSIONS AND IMMUNITY ........................................................................................... 93
SYSTEM PRODUCTS, COMPONENTS, ACCESSORIES, AND TOOLS ................................................ 98
GLOSSARY .............................................................................................................................................. 101
INDEX ....................................................................................................................................................... 107
3
RNS®System User Manual
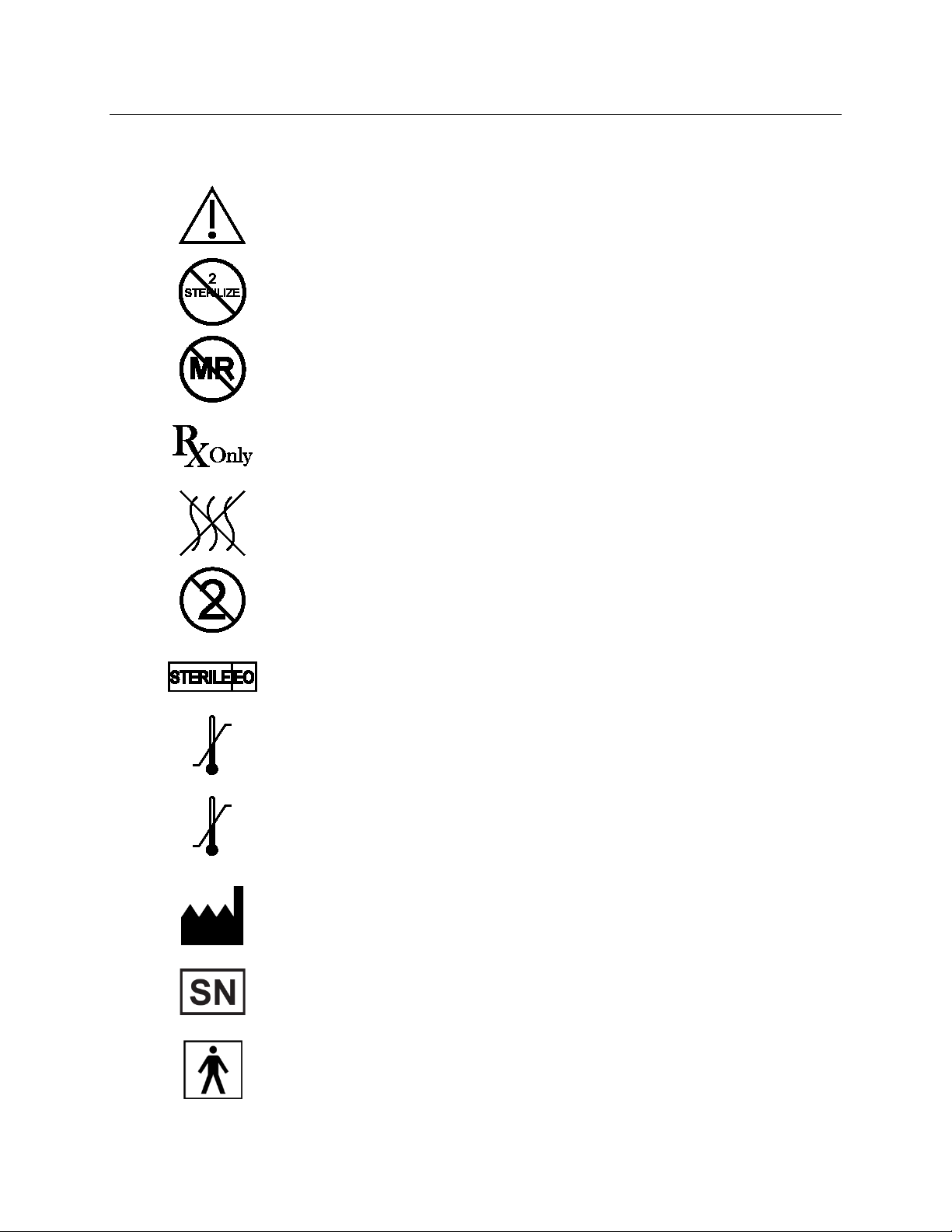
Explanation of symbols on product or package labeling
Refer to the appropriate product for symbols that apply.
Caution
Do Not Resterilize
MR Unsafe
Prescription Only
Non-Pyrogenic
Single Use
Sterilized Using Ethylene Oxide
Use
Temperature limits during use
Storage
Temperature limits during storage or transport
Manufacturer
Serial number
Type BF applied part
4
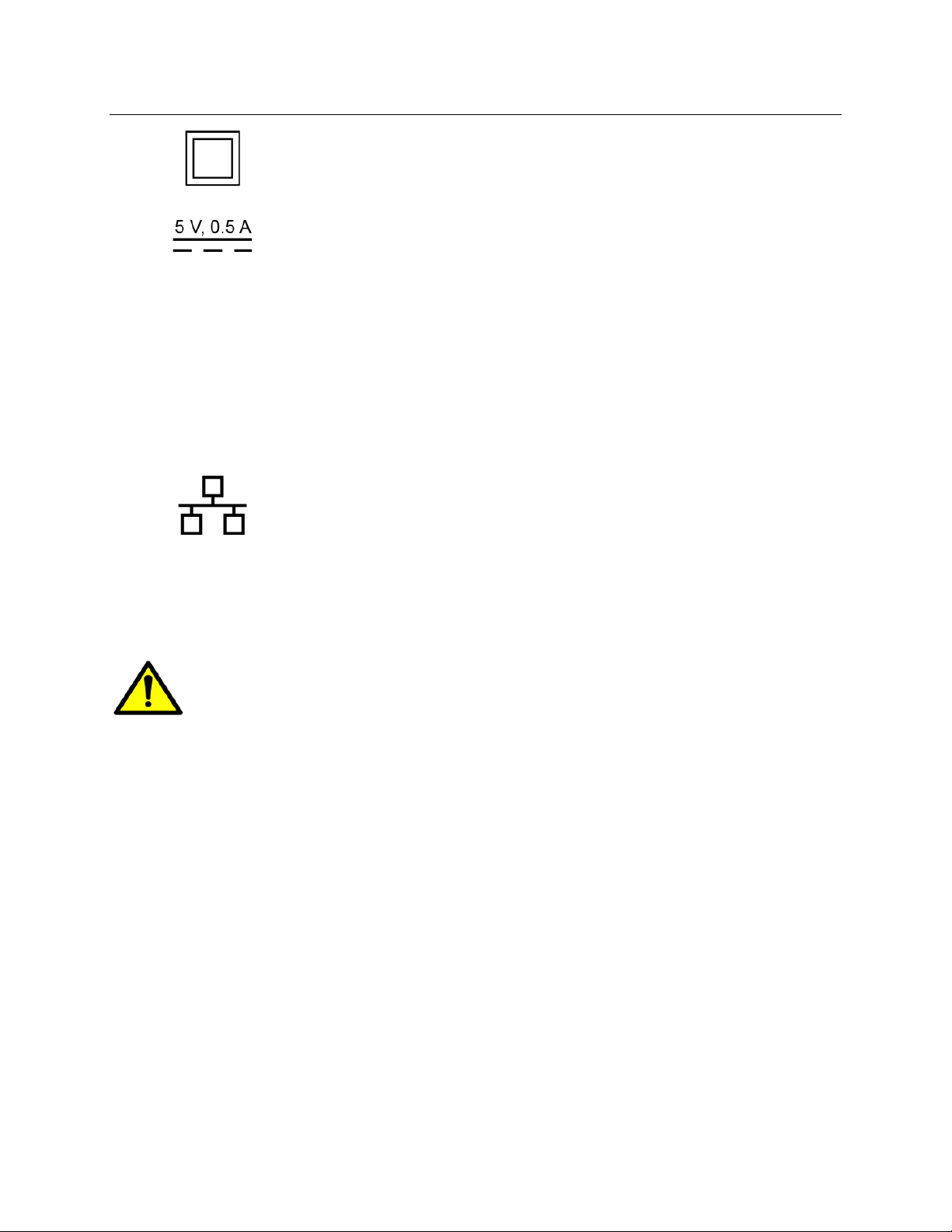
RNS®System User Manual
Class II electrical protection
Direct current (DC) 5 V (volts), 0.5 A
(amperes)
IP22
Ingress protection ratings: Level 2 for solid
objects, which means testing confirms the
device enclosure prevents ingress (entry) of
items greater than 12.5 mm (~1/2 inch), such
as fingers or similar objects; Level 2 for liquids,
which means testing confirms vertically
dripping water shall have no harmful effect
when the enclosure is tilted at an angle up to
15° from its normal position.
Ethernet Connection (Network Connection)
Proposition 65, a State of California voter initiative, requires the following notice:
WARNING: This product can expose you to chemicals including ethylene oxide, which is
known to the State of California to cause cancer and birth defects or other reproductive harm.
For more information go to www.P65Warnings.ca.gov.

Introduction 5
RNS®System User Manual
INTRODUCTION
CONTACTING NEUROPACE
All questions or concerns regarding the NeuroPace®RNS®System should be forwarded to:
NeuroPace, Inc.
455 N. Bernardo Ave.
Mountain View, CA 94043
Customer Support: 1-866-726-3876
(Toll Free in the United States)
Fax: 650-237-2855
Website: www.NeuroPace.com
ABOUT THIS MANUAL
This manual is intended to provide guidelines for implanting the RNS®System, including the
NeuroPace®RNS®Neurostimulator, NeuroPace®Cortical Strip Leads and NeuroPace®Depth Leads,
and instructions for using the NeuroPace®Programmer.
Manual Contents
•Introduction - Overview of the typographical conventions and contents of the manual
•RNS®System Description - Indications, contraindications, product descriptions, theory of
system operation, warnings, and precautions
•Clinical Use of the RNS®System - Physician and center access, identifying candidates,
pre-surgical planning, patient training, overview of implantation and initial programming
recommendations
•Surgical Procedures - Instructions for implanting the RNS®System
•Instructions for Use - Description of the settings available in the neurostimulator and
instructions for programming, instructions for individualizing patient detection settings,
instructions for individualizing patient therapy settings
•Patient Follow-Up - Guidelines for follow-up appointments, follow-up activities, and patient
counseling
•Troubleshooting - Information that may be helpful in solving problems encountered while
implanting or operating the RNS®System
•Specifications - List of each product with its mechanical and electrical characteristics
•Glossary - Alphabetical list of terms used in the manual with their definitions

Introduction 6
RNS®System User Manual
Typographic Conventions
This manual uses different formats and symbols to distinguish instructions, warnings, precautions,
notes, lists, and figures.
WARNING: WARNING TITLE
Warnings alert the user to serious adverse events and potential safety hazards and
situations that may cause injury.
Precaution: PRECAUTION TITLE
Precautions alert the user to exercise special care in the safe and effective use of the
RNS®System.
Note: Notes provide additional information that is particularly useful or important.
1. Numbered paragraphs contain instructions that provide explanations and/or procedural
information.
•Bullet points indicate items in a list.
Figure: Statements regarding a figure are located below the figure between double lines such as this.
Bold italicized text refers the user to a specific location in this manual for further details.
BOLD SMALL CAPS assist the user in navigating to the appropriate tab or button on the NeuroPace®
Programmer screen.
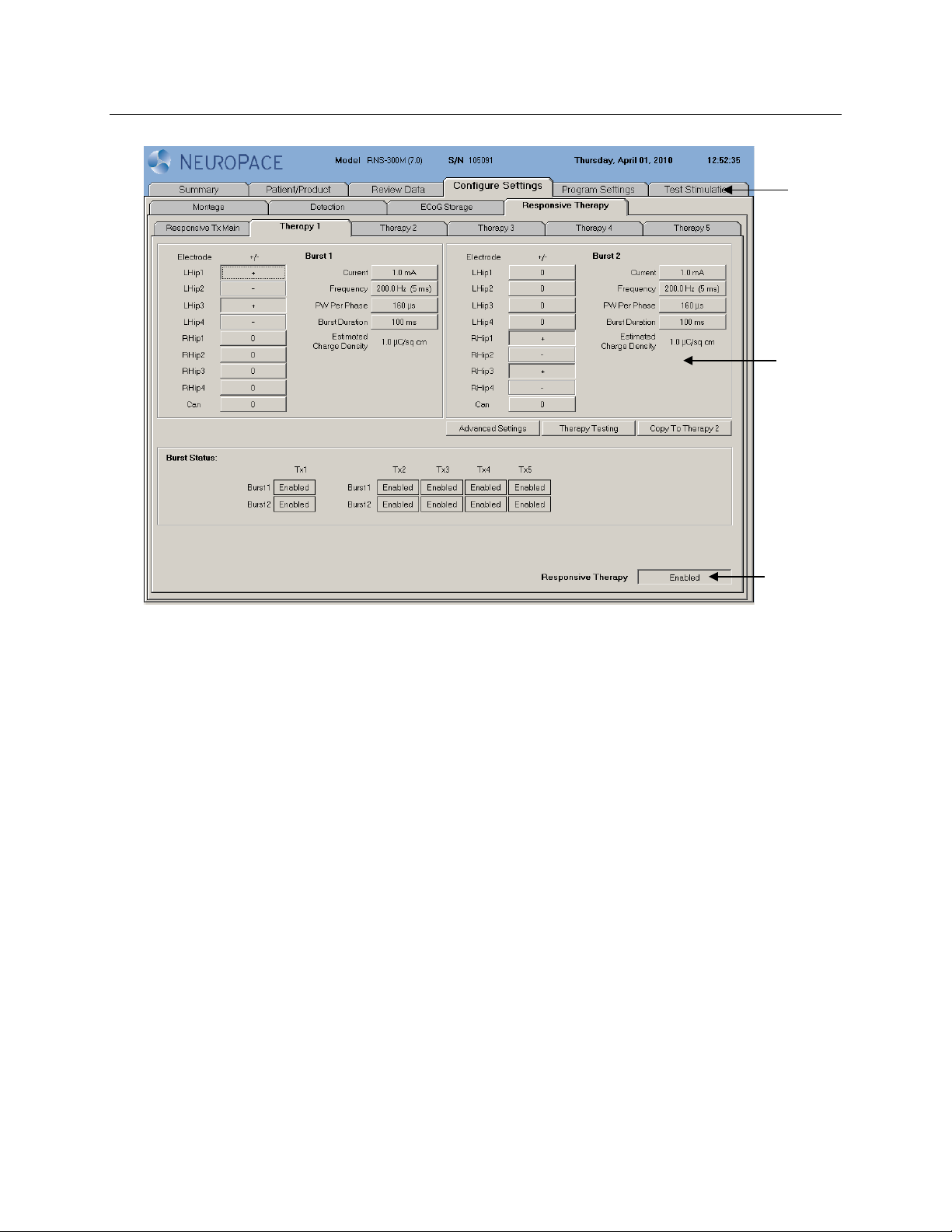
Introduction 7
RNS®System User Manual
The tabs are displayed on the NeuroPace®Programmer screen as follows:
Tab
Page
Button

Responsive Neurostimulator System 8
RNS®System User Manual
RNS®SYSTEM
INDICATIONS
The RNS®System is an adjunctive therapy in reducing the frequency of seizures in individuals 18
years of age or older with partial onset seizures who have undergone diagnostic testing that localized
no more than 2 epileptogenic foci, are refractory to two or more antiepileptic medications, and
currently have frequent and disabling seizures (motor partial seizures, complex partial seizures and /
or secondarily generalized seizures). The RNS®System has demonstrated safety and effectiveness
in patients who average 3 or more disabling seizures per month over the three most recent months
(with no month with fewer than two seizures), and has not been evaluated in patients with less
frequent seizures.
CONTRAINDICATIONS
The RNS®System is contraindicated for:
•Patients at high risk for surgical complications such as active systemic infection, coagulation
disorders (such as the use of anti-thrombotic therapies) or platelet count below 50,000.
•Patients who have medical devices implanted that deliver electrical energy to the brain.
•Patients who are unable, or do not have the necessary assistance, to properly operate the
NeuroPace®Remote Monitor or magnet.
The following medical procedures are contraindicated for patients with an implanted RNS®System.
Energy from these procedures can be sent through the implanted brain stimulation system and cause
permanent brain damage which may cause severe injury, coma, or death. Brain damage can occur
from any of the listed procedures even if the RNS®Neurostimulator is turned off or if the leads are not
connected to the neurostimulator, and can occur even if the neurostimulator has been removed and
any leads (or any part of a lead) or the cranial prosthesis remain.
•MR imaging is contraindicated for patients with an implanted RNS®System. Do not perform an
MRI on a patient with any implanted RNS®Neurostimulator or lead (or any portion of a lead).
Even if the neurostimulator has been removed, you should not have an MRI if any part of a lead
or the cranial prosthesis is still implanted.
The RNS®System is MR Unsafe. Testing has not been performed to define conditions of use to
ensure safety of the RNS®System in an MR environment.
•Diathermy procedures are contraindicated in patients implanted with an RNS®Neurostimulator
and associated leads. (Diathermy is any treatment that uses high-frequency electromagnetic
radiation, electric currents, or ultrasonic waves to produce heat in body tissues.) Patients
absolutely CANNOT be treated with any type of shortwave, microwave, or therapeutic ultrasound
diathermy device whether or not it is used to produce heat. These treatments should not be
applied anywhere on the body.
•Electroconvulsive Therapy (ECT) is contraindicated for patients with an implanted RNS®System.
•Transcranial Magnetic Stimulation (TMS) is contraindicated for patients with an implanted RNS®
System.
WARNINGS AND PRECAUTIONS
WARNINGS -CLINICAL USE
WARNING: PHYSICIAN AND CENTER ACCESS TO THE RNS®SYSTEM
The RNS®System should only be implanted by neurosurgeons with adequate
experience in the implantation of subdural and stereotactic implantation of
intraparenchymal electrodes and in the surgical treatment of intractable epilepsy. The

Responsive Neurostimulator System 9
RNS®System User Manual
RNS®System should only be used by neurologists or neurosurgeons with adequate
experience in the management of intractable epilepsy and in the localization of
epileptic foci, including the use of scalp and intracranial electrodes.
Neurologists and neurosurgeons using the RNS®System must have completed the
NeuroPace®RNS®System training program. To qualify to manage patients with the
RNS®System, physicians must demonstrate specific expertise related to epilepsy,
video-EEG monitoring, interpretation of electrocorticograms (ECoGs), the
pharmacology of antiepileptic medications and selection of patients for epilepsy
surgery. Implantation of the RNS®System should be performed only by qualified
neurosurgeons at centers capable of providing comprehensive epilepsy care, i.e.
“Comprehensive Epilepsy Centers”. These centers should have the expertise to
provide diagnostic services that include video-EEG monitoring with scalp and
intracranial electrodes and neuroimaging, and are experts in the treatment of epilepsy
with antiepileptic medications, epilepsy surgery and devices.
WARNING: MANAGEMENT OF PATIENTS WITH THE RNS®SYSTEM BY PHYSICIANS AT CENTERS THAT DO
NOT PROVIDE THE SERVICES PROVIDED AT COMPREHENSIVE EPILEPSY CENTERS
In some instances, post-implant programming may be conducted by neurologists
meeting the experience and certification requirements for neurologists at
Comprehensive Epilepsy Centers, but who are not practicing in such centers. This
situation might occur if the patient is not able to travel to a Comprehensive Epilepsy
Center for regular follow-up (e.g. because of distance from the Center or limited
access to transportation). These neurologists will be qualified by NeuroPace to provide
post-implant programming. After NeuroPace®RNS®System training is complete, the
qualified programming neurologist may receive external NeuroPace products
(programmer, remote monitor).
WARNINGS –SURGICAL
WARNING: COMPATIBILITY WITH SIMILAR IMPLANTABLE PRODUCTS
The NeuroPace®RNS®Neurostimulator, NeuroPace®Cortical Strip Lead, and
NeuroPace®Depth Lead are not compatible with non-NeuroPace leads and/or pulse
generators. Incompatible configurations may cause damage to the products and may
result in unsafe current densities delivered to the patient.
WARNING: CORTICAL STRIP LEAD EXPLANTATION
Explanting a chronically implanted cortical strip lead may cause tissue damage.
WARNING: INFECTION
Infection, including bacterial meningitis, may occur as a result of the RNS®System
implant procedures and/or the RNS®System materials. Standard surgical infection
prevention measures (antibiotics etc.) should be taken both pre- and post-implantation.
WARNING: INTRACRANIAL HEMORRHAGE
Intracranial hemorrhage may occur when implanting the RNS®System. Placing the
leads, ferrule, and/or neurostimulator in an area where excess pressure may occur to
the underlying blood vessels may cause intracranial hemorrhage. Patients with
underlying risk factors for intracranial hemorrhage, such as patients with previous

Responsive Neurostimulator System 10
RNS®System User Manual
head trauma, anticoagulant use, or who experience head injury with seizures should
be taken into specific consideration.
WARNING: SURGICAL PROCEDURE SIDE EFFECTS
Surgical procedure risks may include, but are not limited to, temporary pain at the
implant site, CSF leakage and although rare, epidural hemorrhage, seroma, subdural
or epidural hematoma and paralysis.
WARNINGS –RNS®SYSTEM AND THERAPY
WARNING: ADVERSE TISSUE REACTION
Allergic reaction to the RNS®System materials and/or leads implanted is possible.
WARNING: CHRONIC TISSUE STIMULATION
The effects of long-term brain stimulation are not completely known and may present
some risks to the patient.
WARNING: EROSION
Skin erosion may occur on and/or around the neurostimulator and/or lead implant site,
particularly in the case of protrusion of the implanted RNS®System products above
the surface of the skull.
WARNING: LEAD MIGRATION
The implanted lead(s) may migrate from their desired implant location. Lead migration
can result in changes in detections and stimulation effectiveness, and may require
additional surgical procedures to modify the lead location.
WARNING: PREGNANT WOMEN
The safety and effectiveness of the RNS®System has not been studied in pregnant
women.
WARNING: RNS®SYSTEM FAILURE
As with any electronic device, the RNS®System may malfunction (not work). Potential
causes include battery malfunctions, an electrical short, open circuits, lead fractures,
lead insulation failures, or damage as a result of head trauma. These malfunctions are
unpredictable, and may result in too little stimulation or no stimulation. A lead failure
may result in the lead needing to be removed or repositioned, which would require
surgery. A malfunctioning neurostimulator may need to be replaced, which would
require surgery. Although the neurostimulator is designed to turn off if overstimulation
or excess current occurs, there is a possibility that product failure could result in brain
tissue damage.
WARNING: PATIENT DATA COLLECTION
The patient must be willing to collect data daily from their neurostimulator and send the
data to the PDMS database at least once a week.
WARNING: CASE DAMAGE
If the neurostimulator case is ruptured or pierced due to outside forces, severe brain
tissue damage could result from exposure to the battery chemicals.
WARNING: ELECTROMAGNETIC INTERFERENCE (EMI)

Responsive Neurostimulator System 11
RNS®System User Manual
Electromagnetic interference is a field of energy generated by equipment found in the
home, work, medical, or public environments that is strong enough to interfere with
neurostimulator function. Sources of strong electromagnetic interference can result in
the following effects:
•Serious patient injury or death - It is possible for the interference sources to
couple enough energy into a neurostimulator system to damage brain tissue
•System damage - resulting in a loss or change in symptom control and requiring
reoperation
•Operational changes to the neurostimulator - causing stimulation to turn on or
off, or resetting or reprogramming the neurostimulator resulting in a return of
symptoms
•Unexpected changes in stimulation - causing a momentary increase in
stimulation which may be felt by the patient
Patients should exercise caution in avoidance of devices which generate a strong
electric or magnetic field. Refer to Electromagnetic Emissions and Immunity for
more information.
WARNING: RADIO FREQUENCY IDENTIFICATION (RFID) INTERFERENCE
Sources of RFID can result in signals that appear as ECoG activity to the
neurostimulator. Signals that appear as ECoG activity could also result in delivering
the programmed stimulation to the patient (per the device detection programming).
The physician should be aware of possible sensing artifacts when assessing the
ECoG recordings. Potential sources of RFID may occur in a health care environment,
retail stores, public libraries, airports and business environments.
Refer to Electromagnetic Emissions and Immunity for more information.
WARNING: SECURITY AND ELECTRONIC TRACKING SYSTEMS
Security screening devices (such as theft detectors and airport security screening
devices) can result in signals that appear as ECoG activity to the neurostimulator.
Signals that appear as ECoG activity could also result in delivering the programmed
stimulation to the patient (per the device detection programming). Such devices may
be found at retail stores, public libraries and airports. The physician should be aware
of possible sensing artifacts when assessing the ECoG recordings. Patients should be
instructed to walk through the center of such security screening units without stopping,
when possible, and exit the area of the screening device as soon as possible.
Refer to Electromagnetic Emissions and Immunity for more information.

Responsive Neurostimulator System 12
RNS®System User Manual
WARNING: INTERACTION WITH IMPLANTED CARDIAC DEVICES
Possible effects of implanted device interaction with an implanted cardiac device (e.g.,
pacemaker or defibrillator) include the following:
•Defibrillation therapy from an implanted defibrillator may damage the device.
•The electrical pulses from the neurostimulation system may interact with the
sensing operation from a cardiac device and could result in an inappropriate
response of the cardiac device and vice versa.
WARNINGS –PROGRAMMER
WARNING: POTENTIAL SHOCK
Submerging any part of the programmer, or operating the programmer in or near a wet
environment, may result in an electrical shock.
The programmer must be disconnected from the electrical outlet prior to cleaning to
avoid the potential of electrical shock.
Electrical shock may occur if the programmer AC adapter and power cord are not
properly connected to a grounded power source.
Do not attempt to modify or repair the wand or programmer. Contact NeuroPace
Customer Support for assistance.
WARNING: NEUROPACE COMPONENTS
Use of accessories, transducers, and cables other than those provided by NeuroPace
could result in increased electromagnetic emissions or decreased electromagnetic
immunity of the RNS® System and result in improper operation. Do not plug the wand
into equipment other than the programmer because it could damage the wand. Do not
use a USB cable extension from the programmer to the wand.
WARNING: PORTABLE RADIO FREQUENCY (RF) COMMUNICATIONS EQUIPMENT
Portable RF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 12 inches (30 cm) to any part of
the RNS® System, including cables. Otherwise, degradation of the performance of the
RNS® System could result.
WARNING: NEUROPACE®EQUIPMENT PLACEMENT
Use of NeuroPace®equipment (for example, remote monitor or programmer) adjacent
to or stacked with other equipment should be avoided because it could result in
improper operation. If such use is necessary, the NeuroPace®equipment and other
equipment should be observed to verify that they are operating normally.
WARNINGS –MEDICAL ENVIRONMENT
WARNING: LITHOTRIPSY
The effects of extracorporeal shock wave lithotripsy on the RNS®System have not
been studied. Exposure to high-output ultrasonic frequencies may damage the RNS®

Responsive Neurostimulator System 13
RNS®System User Manual
System. This could result in loss of therapy, and additional surgery to remove or
replace components of the RNS®System. Prior to any administration of lithotripsy, the
administering physician should consult with the physician prescribing the RNS®
System.
WARNING: RADIATION
The effects of high radiation sources (such as cobalt 60 or gamma radiation used in
cancer therapy) on the RNS®System have not been studied. Exposure to high levels
of radiation may damage the RNS®System. This could result in loss of therapy, and
additional surgery to remove or replace components of the RNS®System. Prior to any
course of radiation therapy, the radiation oncologist should consult with the physician
prescribing the RNS®System.
WARNING: ELECTROLYSIS
The effects of electrolysis on the RNS®System have not been studied. Electrolysis on
the head or neck should be avoided.
WARNING: COMPUTERIZED TOMOGRAPHY (CT) SCANS
For CT procedures on a patient with an implanted RNS®Neurostimulator, the operator
should:
•Ask the patient to have the neurostimulator temporarily shut off with a programmer
while the scan is performed, if possible.
•Minimize x-ray exposure to the implanted electronic medical device by:
•Using the lowest possible x-ray tube current consistent with obtaining the required
image quality.
•Making sure that the x-ray beam does not dwell over the device for more than a
few seconds.
Important note: For CT procedures that require scanning over the medical device
continuously for more than a few seconds, as with CT perfusion or interventional
exams, attending staff should be ready to take emergency measures to treat adverse
reactions if they occur.
After CT scanning, the operator should:
•Ask the patient to have the neurostimulator turned back on with a programmer if it
had been turned off prior to scanning.
•Advise the patient to contact their healthcare provider as soon as possible if they
have questions or suspect their device is not functioning properly after any medical
procedure.

Responsive Neurostimulator System 14
RNS®System User Manual
PRECAUTIONS –SURGICAL
Precaution: Connector Plug
A vacant port in the connector cover must be filled with a connector plug (provided in
the connector cover kit). There is an increased risk of neurostimulator failure if a
connector cover port is vacant.
Precaution: Epidural Lead Placement
Leads placed epidurally may cause pain during electrical stimulation.
Precaution: Lead Damage
Bending, kinking, and stretching of the lead may cause lead damage. Handle the lead
with care.
Precaution: Sub-galeal Lead Placement
Wrapping the lead(s) on/around the neurostimulator or placing excess lead near the
neurostimulator may result in lead damage during subsequent surgical procedures.
Precaution: Suture Sleeves
Suture sleeves are provided for use if sutures are used to stabilize the lead. Suturing
directly on the lead may cause lead body damage and malfunction.
PRECAUTIONS –RNS®SYSTEM AND THERAPY
Precaution: Afterdischarge Activity
If evidence of afterdischarge activity resulting from stimulation is seen either on stored
ECoGs or during test stimulation delivery, stimulation parameters should be adjusted
to prevent such occurrence.
Precaution: Battery Depletion
For continued operation, the RNS®Neurostimulator needs to be surgically replaced
when the battery is depleted.
Precaution: Neurostimulator Longevity
High and frequent levels of stimulation reduce neurostimulator battery longevity.
Precaution: Draining the Neurostimulator Battery
Testing the wand placement over the RNS®Neurostimulator for more than 10 minutes
per day may drain the neurostimulator battery prematurely.
Precaution: Frequency of Remote Monitoring
The patient should interrogate the RNS®Neurostimulator with the remote monitor and
wand daily and synchronize the remote monitor with the PDMS at least once a week.

Responsive Neurostimulator System 15
RNS®System User Manual
Precaution: Explantation and EMI Considerations
If any system components (neurostimulator, leads, lead fragments, or cranial
prosthesis) remain implanted in the patient after a partial system explant, the patient is
still susceptible to possible adverse effects from strong sources of EMI. It is possible
for the interference sources to couple enough energy into a neurostimulator system to
damage brain tissue, resulting in serious patient injury or death.Patients who have
system components implanted should exercise caution in avoidance of devices which
generate a strong electric or magnetic field.
Refer to Electromagnetic Emissions and Immunity for more information.
Precaution: Lead Replacement and Abandoned Leads
The long-term safety associated with leads left in place without use, replacement of
leads, and lead explant is unknown.
PRECAUTIONS –PROGRAMMER
Precaution: Programmer Failure
As with any electronic device, the programmer may be damaged or malfunction if the
programmer AC adapter and power cord are not properly connected to a grounded
power source.
PRECAUTIONS –MEDICAL ENVIRONMENT
Precaution: Medical Procedures
Patients should always inform any healthcare personnel that they have an implanted
RNS®System (and show their medical implant identification card) before any
procedure is performed.
Advise the patient to contact their healthcare provider as soon as possible if they have
questions or suspect their device is not functioning properly after any medical
procedure.
Precaution: Electrocautery
The use of electrocautery (electrosurgery) can affect the operation of neurostimulators.
The RNS®System has been designed to prevent or minimize the effects of
electrocautery, however the energy levels used in electrocautery can temporarily
interfere with or cause permanent damage to device operation.
Electrocautery applied near the RNS®Neurostimulator may cause it to temporarily
stop sensing, delivering therapy, or may reset the neurostimulator. Under these
conditions the neurostimulator may require interrogation and possible reprogramming.
Electrocautery applied directly to the neurostimulator or leads may couple enough
energy into a neurostimulator system to damage brain tissue.
If electrocautery is necessary, the following recommendations may be effective in
minimizing potential complications.
Before the procedure:

Responsive Neurostimulator System 16
RNS®System User Manual
•If possible, temporarily disable stimulation using a programmer.
During the procedure:
•Use of bipolar electrocautery is recommended and should be considered, whenever
possible.
•Keep the electrocautery tip more than 2 cm (approximately one inch) from the
implanted device.
•The selected output power of the electrocautery unit should be as low as possible
for the relevant application and not used for greater than 10 seconds in any one
burst.
After the procedure:
•If stimulation was temporarily disabled before the procedure, re-enable stimulation
with the programmer and synchronize the programmer with the PDMS.
•Advise the patient to contact their healthcare provider as soon as possible if they
have questions or suspect their device is not functioning properly after any medical
procedure.
Precaution: Dental Therapy and Procedures
Dental therapy and procedures that do not involve any of the procedures in the
contraindications or warnings sections of this manual should be performed with
caution. The dentist or dental technician should be informed that the patient is
implanted with the RNS®System.
Advise the patient to contact their healthcare provider as soon as possible if they have
questions or suspect their device is not functioning properly after any medical
procedure.
The following medical procedures may be performed without affecting the RNS®
System:
•Diagnostic x-rays
•Diagnostic ultrasound
Precaution: Other Active Implanted Medical Devices
RNS®System interactions with other active implantable medical devices (such as
pacemakers, defibrillators, implanted spinal cord and peripheral nerve stimulators,
cochlear implants, and vagus nerve stimulators) are not known. Exercise caution when
other implanted devices are operating concurrently with the RNS®System. Possible
effects include sensing problems and inappropriate device responses.
Precaution: Incompatibility of NeuroPace®Programmer with Other Medical Devices
The effects of using the NeuroPace®Programmer to interrogate other electronic,
programmable devices such as pacemakers, defibrillators, cochlear implants, and
other neurostimulators or CPAP machines are unknown. It could result in
reprogramming of the other device and therefore, the physicians familiar with each
device should check the programmed parameters of each device before the patient is
discharged and after each programming session of either device.

Responsive Neurostimulator System 17
RNS®System User Manual
Precaution: Electronic Interference
Communications between the programmer and the implanted neurostimulator may be
interrupted by emissions from nearby electronic devices. Examples of sources of EMI
are lithotripsy, computer monitors, cellular telephones, motorized wheel chairs, x-ray
equipment and other monitoring equipment. Interruption of telemetry can result in
incomplete communication. If EMI disrupts programming, move the programmer away
from the likely source of EMI. Refer to the Poor or No Communication Between the
RNS® Neurostimulator and the Programmer section for more information.
Precaution: Placement of the Programmer Power Cords
Make sure nothing rests on the programmer power cable and that the cable is not
located where it can be tripped over or stepped on.
Precaution: Heating
The programmer AC adapter and the bottom of the laptop computer may become hot
during normal operation. Use care when handling the adapter during or immediately
after operation.
PRECAUTIONS –HOME OR OCCUPATIONAL
Precaution: Keep Magnets at least 4 inches Away from the Implanted RNS®Neurostimulator
Magnets that are contained in such products as stereo speakers, AM/FM radios,
power tools, cellular, cordless and conventional phones, as well as magnets used
therapeutically or worn on the body, should be kept at least 4 inches away from the
neurostimulator. The neurostimulator may not deliver stimulation while these magnets
are closer than 4 inches. Most headsets and earphones available in stores do not
interfere with the RNS®System, but not all have been tested.
Precaution: Magnet
Use care when handling the magnet as it may break if dropped and the broken pieces
may have sharp edges.
Precaution: Scuba diving or hyperbaric chambers
Patients should not dive below 10 meters (33 feet) of water or enter hyperbaric
chambers above 2.0 atmospheres absolute (ATA). Such pressures could damage the
system.
Precaution: Patient Population for which safety and efficacy have not been established
The safety and effectiveness of the device has not been established for the following:
•Patients with generalized epilepsy
•Patients with a seizure focus that cannot be adequately localized
•Pregnant women
•Nursing mothers
•Less than age 18
•Patients with simple partial sensory seizures only
•Patients with less than three seizures a month on average
•Patients who have more than two epileptic foci
•Patients who have not failed two antiepileptic drugs
Precaution: Safety and Effectiveness beyond 24 months
The safety and effectiveness of the RNS®System beyond 24 months is unknown.
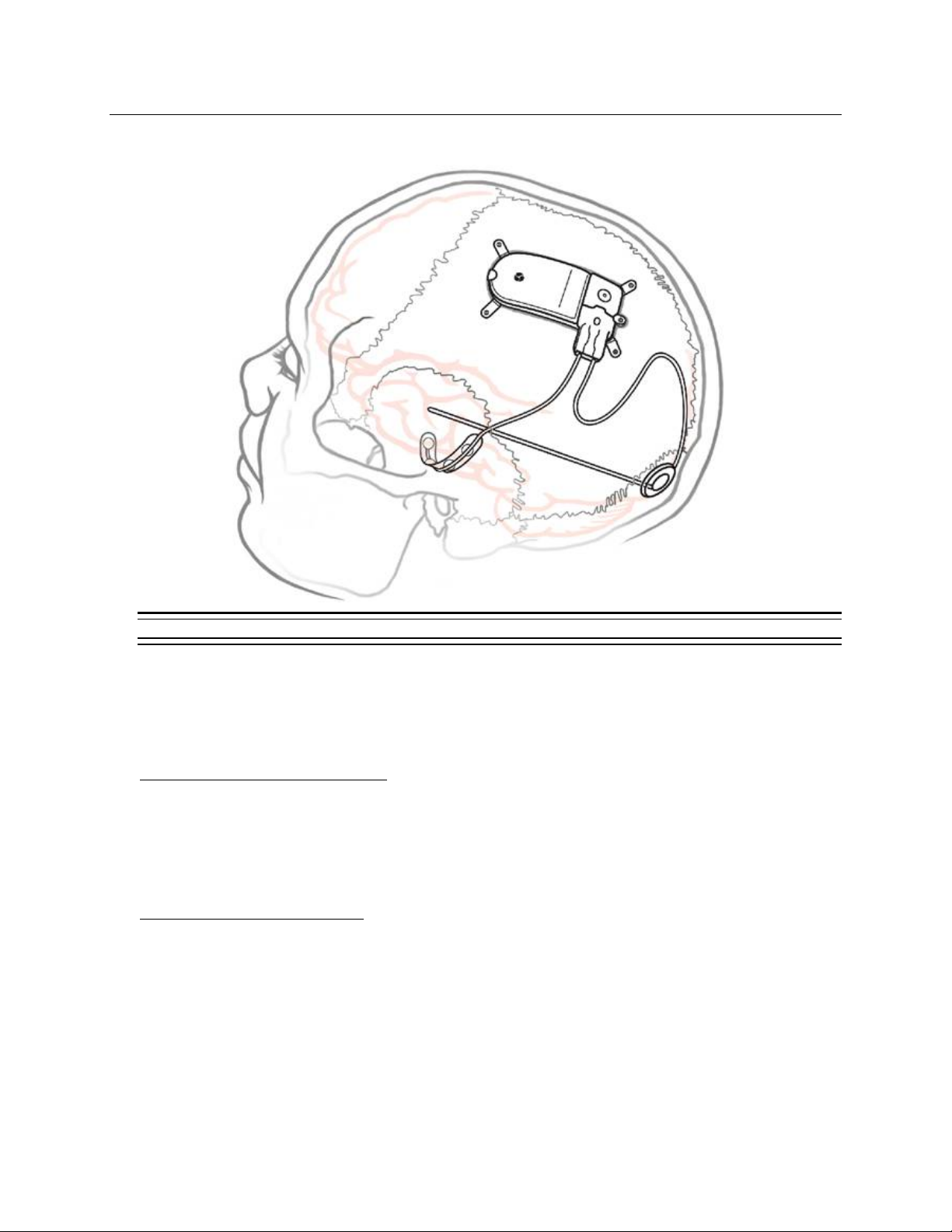
Responsive Neurostimulator System 18
RNS®System User Manual
IMPLANTED RNS®SYSTEM
Figure 1: The Implanted RNS®System.
RNS®SYSTEM DESCRIPTION
The RNS®System automatically delivers responsive electrical stimulation to reduce the frequency of
partial onset seizures. Note that the RNS®System is not a seizure detection device. The RNS®
System comprises sterile, implantable and non-sterile, external products.
Implantable RNS®System Products
The sterile, implantable products are the
•RNS®Neurostimulator
•NeuroPace®Cortical Strip Lead(s) and NeuroPace®Depth Lead(s)
•Implantable components and accessories
External RNS®System Products
The non-sterile, external products are the
•NeuroPace®Programmer (laptop computer with proprietary software) and telemetry
component (wand model W-02) used for communication with the implanted RNS®
Neurostimulator
•NeuroPace®Patient Data Management System (PDMS) used for storage and access to
historical neurostimulator and patient data
This manual suits for next models
9
Table of contents
Other Neuropace Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Biowave
Biowave GO Quick reference guide

Tomey
Tomey AL-100 Service manual

Verathon
Verathon BladderScan BVI 3000 Operator's manual

laerdal
laerdal NeoNatalie Directions for use

Kimberly-Clark
Kimberly-Clark MIC-KEY Low-Profile Jejunal Feeding Tube Directions for use

SamanTree Medical
SamanTree Medical Histolog Scanner Instructions for use