Contents i
W7.091.601 ELECTROSURGICAL GENERATOR ESG-400
Contents
Labels and Symbols.................................................................................................. 4
Important Information - Please Read Before Use ................................................... 5
Intended use .................................................................................................................................... 5
Application of high frequency treatment .......................................................................................... 5
Instruction manual ........................................................................................................................... 5
User qualifications ........................................................................................................................... 6
Electrosurgical generator compatibility............................................................................................ 6
Repair and modification ................................................................................................................... 6
Signal words .................................................................................................................................... 7
Dangers, warnings and cautions ..................................................................................................... 7
Legal information ............................................................................................................................ 16
Chapter 1 Checking the Package Contents......................................................... 17
Chapter 2 Nomenclature and Functions .............................................................. 18
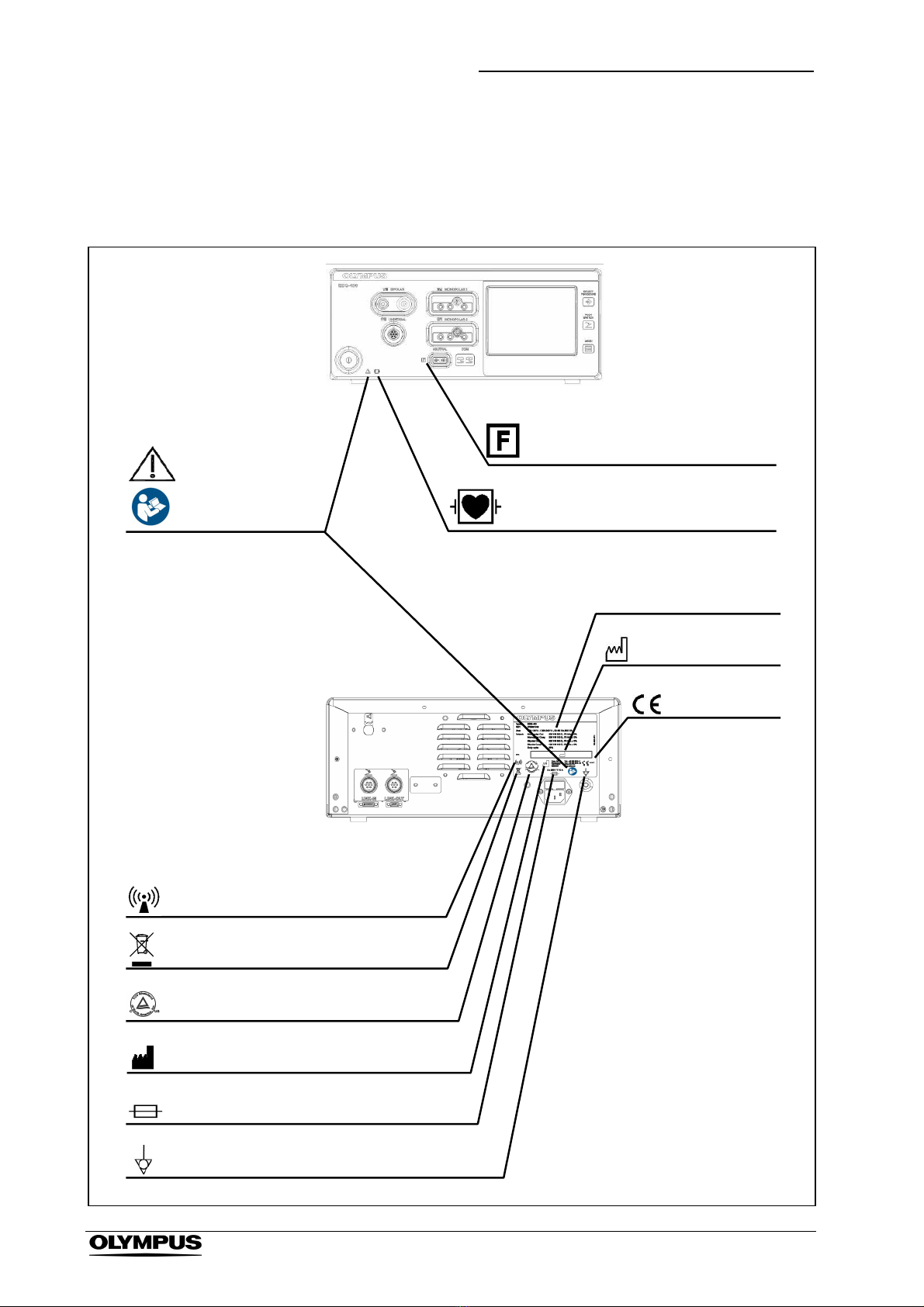
2.1 Symbols and descriptions...................................................................................................... 18
2.2 Front panel ............................................................................................................................ 21
2.3 Rear panel ............................................................................................................................. 23
2.4 Bottom panel ....................................................................................................................... 24
2.5 All screen ............................................................................................................................... 25
2.6 Set screen.............................................................................................................................. 26
2.7 Mode screen .......................................................................................................................... 27
2.8 Footswitch with two pedals.................................................................................................... 27
2.9 Footswitch with one pedal (optional) ..................................................................................... 28
2.10 Neutral electrode cable “P-cord” (optional) ........................................................................... 28
2.11 Communication cable 0.25 m (MAJ-1871, optional, this cable is required for the
connection with the compatible ultrasonic generator) ........................................................... 29
2.12 Communication cable 10 m (MAJ-1872, optional, this cable is required for the
connection with the compatible high flow insufflation unit (UHI-2/3)).................................... 30
2.13 Adapter for UHI-2/3 (MAJ-1873, optional, this adapter is required for the connection
with the compatible high flow insufflation unit (UHI-2/3)) ...................................................... 31
Chapter 3 Installation and Connections .............................................................. 32
3.1 Flow chart for installation work ............................................................................................. 33
3.2 Installation of electrosurgical generator................................................................................. 34
3.3 Connection of peripheral equipment ..................................................................................... 35
3.4 Connection to an AC mains power supply ............................................................................ 36
3.5 Automatic mist & smoke evacuation system/function (when using the compatible
high flow insufflation unit) ...................................................................................................... 37
3.6 Connection of footswitch ....................................................................................................... 42
3.7 Connection of neutral electrode (for monopolar treatment only)........................................... 43
3.8 Connection of HF instruments ............................................................................................... 49