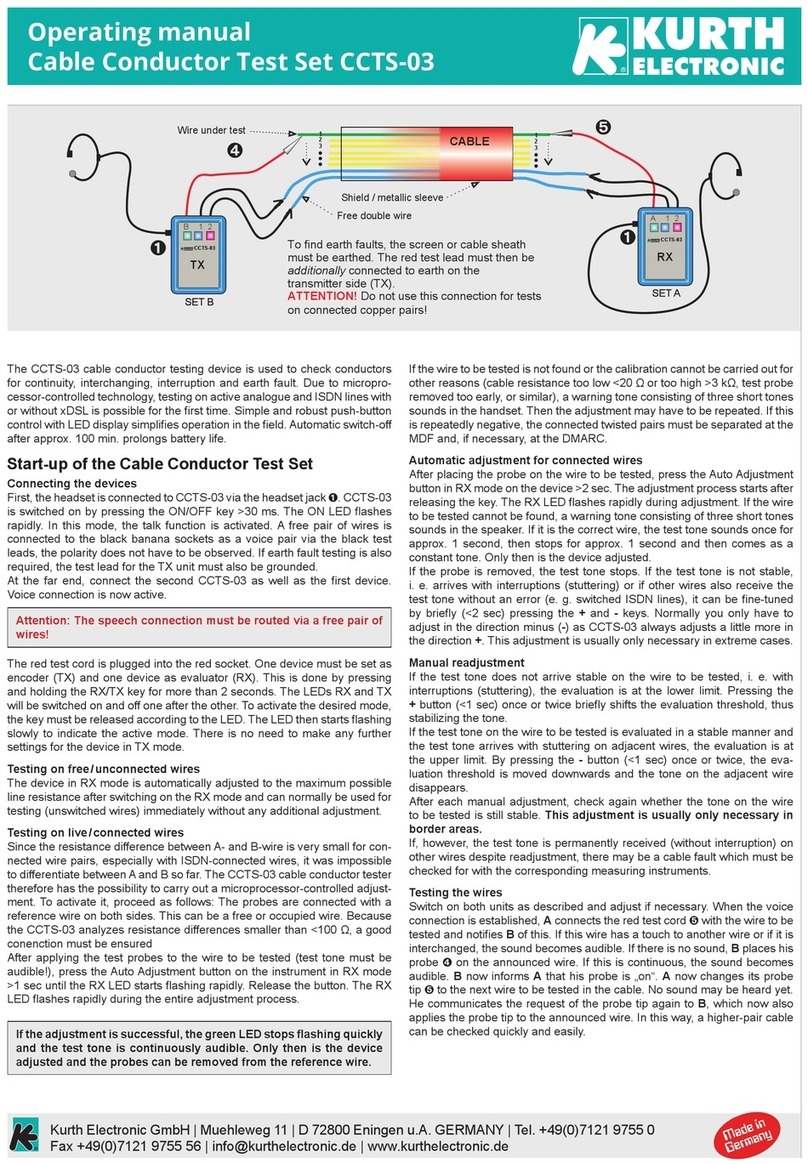
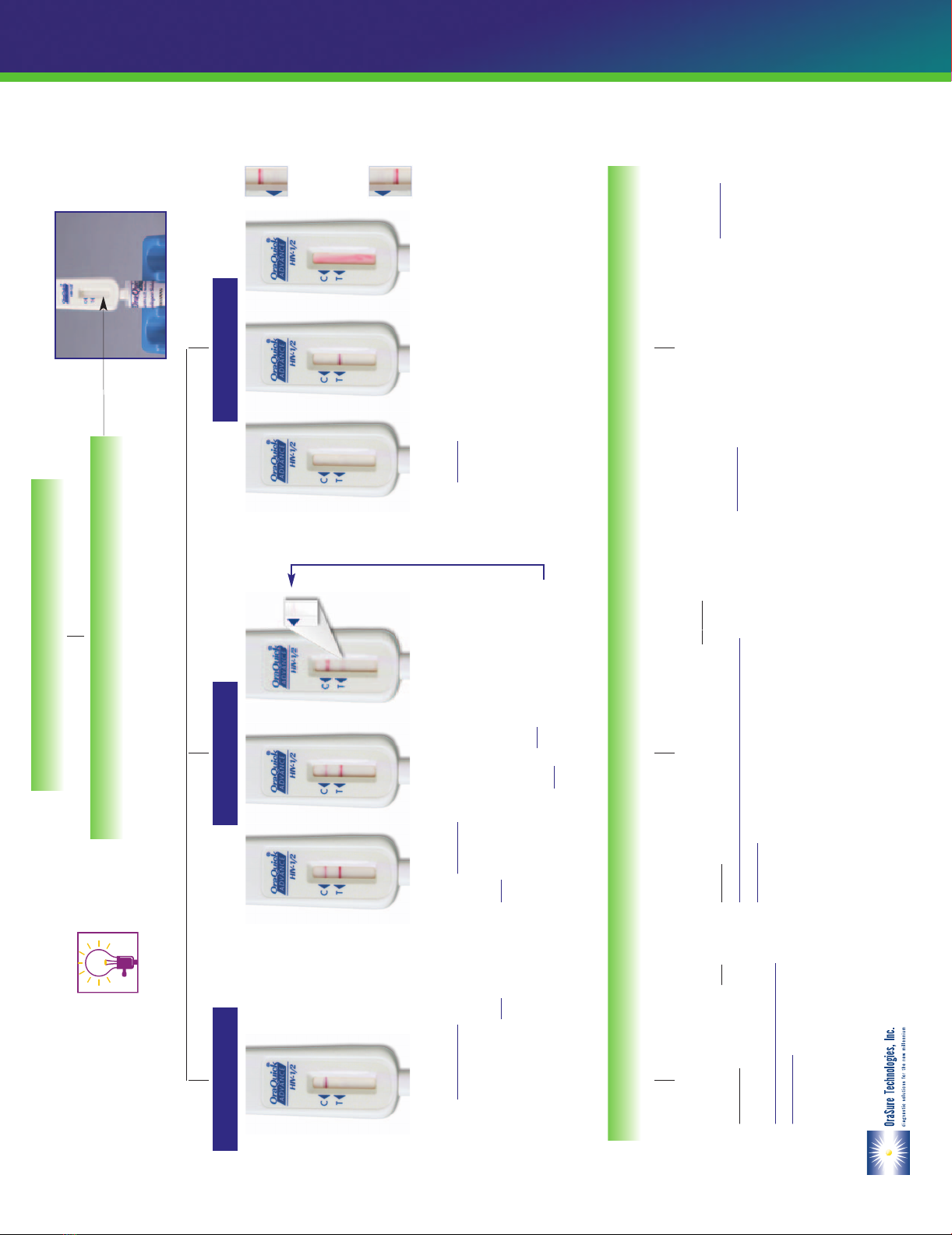
Reading Test Result
Test is INVALID if:
•NO reddish-purple line appears next to the triangle labeled “C”
(see Diagram a and b ), or
•A red background in the Results Window makes it difficult to read the
result after 20 minutes (Diagram c ), or
•If any of the lines areNOTinside the “C” or “T” triangle areas
(see Diagram d1 and d2 ).
•
An Invalid test result means that there was a problem running the test, either
related to the specimen or to the Device. An Invalid result cannot be
interpreted.Repeat the test with a new Pouch and a new oral fluid,
fingerstick or venipuncture whole blood, or plasma sample. Contact
OraSure Technologies’ Customer Service if you are unable to get a valid test
result upon repeat testing.
Test is REACTIVE if:
•A reddish-purple line appears next to the triangle labeled “C”
and a reddish-purple line appears next to the triangle labeled
“T”. One of these lines may be darker than the other.
NOTE: The test is Reactiveif any reddish-purple line appears
next to the “T” triangleand next to the “C” triangle, no matter
how faint these lines are.
•
A Reactivetest result means that HIV-1 or HIV-2 antibodies have been
detected in the specimen. The test result is interpreted as
PRELIMINARY POSITIVE for HIV-1 and/or HIV-2
antibodies. Follow CDC Guidelines to inform the test subject of
the test result and its interpretation.
Test is NON-REACTIVE if:
•
A reddish-purple line appears next to
the triangle labeled “C” and NO
line appears next to the triangle
labeled “T”.
•
ANon-Reactivetest result means
that HIV-1 and HIV-2 antibodies were
not detected in the specimen. The
test result is interpreted as
NEGATIVE for HIV-1 and HIV-
2 antibodies. Follow CDC
Guidelines to inform the test subject
of the test result and its
interpretation.
ADEQUATE
LIGHTING
REQUIRED
abc
d1
OR
d2
Bethlehem, PA 18015 USA • Ph. 800-ORASURE (800-672-7873) • Fax 610.882.3572 • www.orasure.com L3001-1216 (rev. 10/05)
©2001, 2005 OraSure Technologies, Inc.
U.S. Patent # 6,303,081 and various international and U.S. patents pending
OraQuick® is a registered trademark of OraSure Technologies, Inc.
Non-ReactiveReactiveInvalid
Interpretation of Test Result
Read results after 20 minutes but not more than 40 minutes.
Look at the Result Window of the Test Device
OQA Eng_SPA_StepbyStep-10-05 10/31/05 4:16 PM Page 1