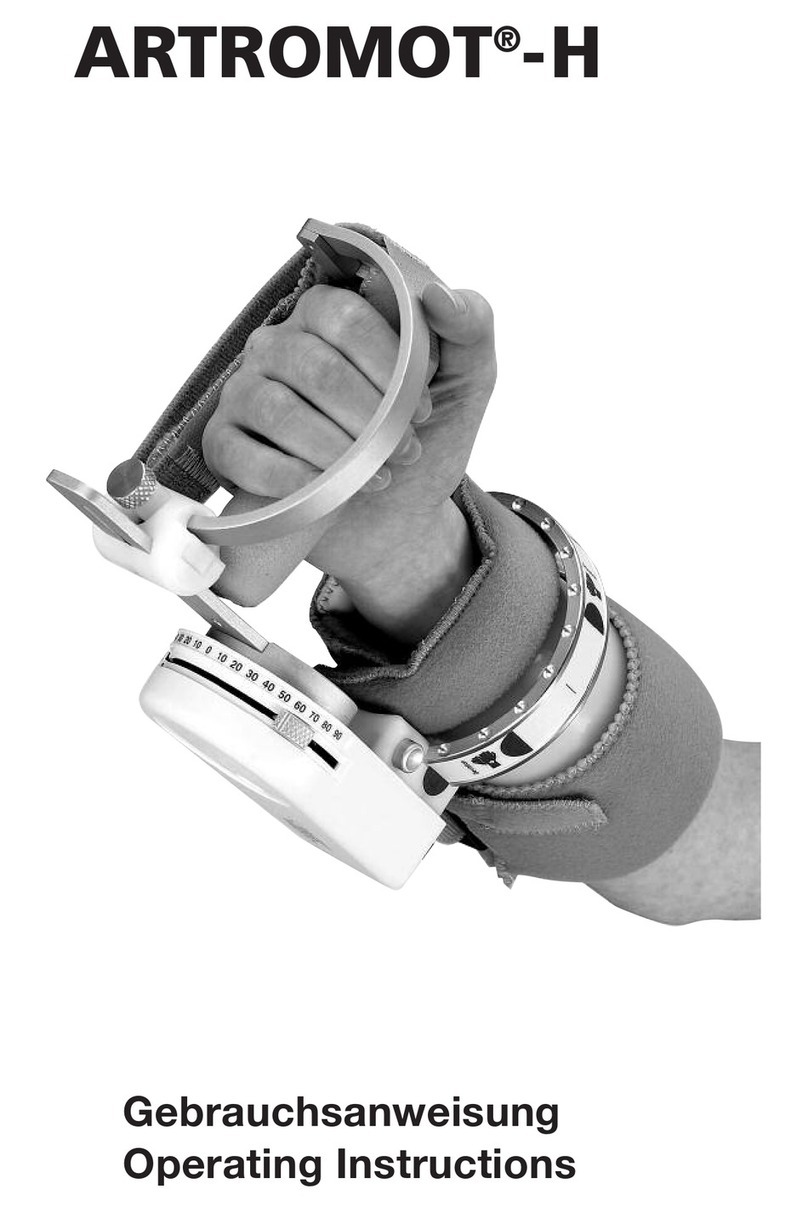
The following treatment values can
be stored by means of the hand-held
programming unit. (7)
– Knee extension
– Knee flexion
– Pause extension
– Pause flexion
– Force
– Speed
IMPORTANT:
It is possible to program single or all
parameters. If only some parametres
were changed, the other parameters
will be saved with current settings.
1. Pressing the Extension and STOP
keys at the same time for one
second or holding down the STOP
key for five seconds enables you to
change to programming mode.
1110
Warm up allows the patient gradually
attain full programmed range of motion.
The device starts in the middle between
the two values set for extension and
flexion. With each movement cycle the
extent of movement is increased by
2 degrees until the set value is reached.
The device then moves between these
values.
The full speed & motion function is only
for service.The device runs at twice
the maximum programmable speed to
facilitate a rapid device set up.
WARNING: Do not run the device in
full speed & motion when patient is
in the device!
The individual run time for each treat-
ment.To reset press SET key in the
programming mode.
The total device run time is counted
from the first usage of the device. Press
+button for 5 seconds until setting
appears. Device run time cannot be
deleted.
To save the programmed special func-
tions, press the STOP key.
Press the START key: the device checks
programmed values.
Adjusting the force (reverse on load)
– Minimum setting for reverse on load:
25 kp
– Maximum setting for reverse on load:
45 kp
Settings are aproximate!
Tensile force is measured on the frame
around the foot.
The input setting determines the maxi-
mum resistance needed to automatical-
ly reverse the direction of motion.
Speed
Minimum setting for speed: 1%
Maximum setting for speed: 100%
Special functions are:
– Center warm up
– Full speed & motion (double speed
setting)
– Runtime (patient runtime)
– Device runtime
Programming the special functions:
1. Switch to programming mode
(section 5.1.1)
2. Press FUNC key
3. Select special funtions using
+or -key
4. Follow the instructions on the display
5. Quit and save with STOP button
2. You can now select the treatment
values in succesion by pressing the
parameter keys.
3. Change the value by pressing the
+/- keys.
4. Continue programming (with 2 and 3)
until all required values are entered.
5. Press the STOP key to save all
previous values.
6. Press START button: programme
values were checked automatically.
7. Press START button again to start
the device in therapy mode.
8. Pressing the parameter buttons
in stop mode the display shows the
current stored values.
Setting the range of motion ROM
–
Maximun knee extension: -10 degrees
– Maximum knee flexion: 125 degrees
The criterion for correct adjustment is
that it should be possible to move the
extremity without pain or irritation.
Adjusting the pauses
– The pauses occur in the final position
of extension of flexion and can be set
seperately for extension and flexion.
– Possible values for pauses:
0–30 seconds.
Fig.1
Buttons to
choose the
parameters:
Extension
Flexion
Pause
Extension
Pause
Flexion
Force
Speed
Display
STOP
START
“SET“
“+” button
“–” button
Function
5. Setting the treatment values
5.1 Programming the
ARTROMOT®-K4
5.1.1 Programming the
treatment values
5.1.2 Information about
treatment values
PRECAUTION!
The programmed value and
the actual angle measured at the
patient´s knee may vary.
Center warm up
Full speed & motion
Run time
Device run time
Save data
PRECAUTION!
The reverse circuit is purely a
safty measure for cramps,spasms,
locked joints, etc. The manufac-
turer accepts no liability if used
improperly.
5.1.3 Programming the
special functions