2
1. Introduction ...................................... 3
2. Intended Use .................................... 3
2.1 Medical Purpose .................................... 3
2.2 Application............................................. 3
2.3 Qualification of Clinician.......................... 3
2.4 Follow up Schedule ................................ 3
3. Indications/Contraindications .... 4
3.1 Indications ............................................ 4
3.2 Contraindications ................................... 4
4. Warnings and Precautions........... 5
4.1 Safety precautions ................................. 5
4.2 Warnings............................................... 5
4.3 General cautions ................................... 7
4.4 Potential Risks/Side effects ..................... 9
5. Service Information...................... 11
5.1 Scope of Delivery ................................. 11
5.2 Service................................................ 11
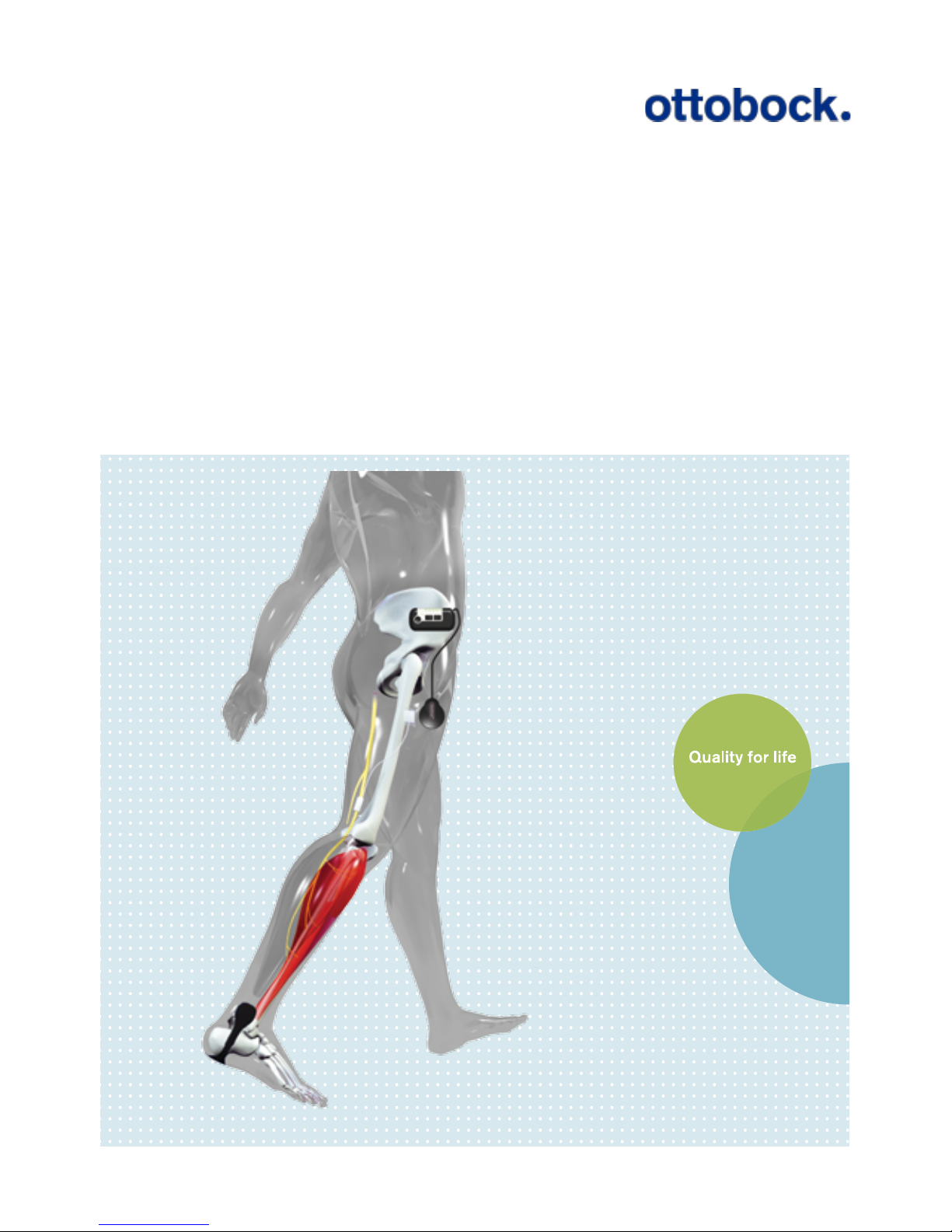
6. Description of the ActiGait®....... 12
6.1 The ActiGait®Implant ........................... 13
6.2 The ActiGait®....................................... 14
7. How ActiGait®works ................... 15
7.1 The Control Unit ................................... 16
7.1.1 The On/Off Button ............................. 17
7.1.2 The Stimulation Intensity Buttons ......... 18
7.1.3 The Mute Button ................................ 19
7.1.4 The Pairing Button ............................. 21
7.1.5 The Control Unit Battery Indicator ........ 22
7.1.6 The Heel Switch Battery Indicator ........ 23
7.1.7 Training Mode Indicator ...................... 25
7.1.8 Mute Indicator ................................... 27
7.1.9 Stimulation Intensity Indicators ............ 27
7.2 Putting On the Control Unit.................... 27
7.2.1 The Belt Clip ..................................... 28
7.2.2 The Belt Loop .................................... 28
7.2.3 The Body Clip ................................... 29
7.3 Putting On the Antenna Fixture............... 30
7.4 Putting On the Antenna ......................... 33
7.5 The Heel Switch ................................... 35
7.5.1 The Heel Sock................................... 36
7.5.2 Heel Switch Feedback........................ 37
7.6 The Charger ........................................ 38
7.6.1 Charging the Control Unit................... 39
7.6.2 Travel Kit for Charger ......................... 41
7.7 Audio Signals and Warnings .................. 42
7.7.1 Warnings .......................................... 42
7.7.2 Audio Signals .................................... 43
8. Lights and Audio Signals............ 43
8.1 How to Interpret the Light Indicators ....... 44
8.2 How to Interpret the Audio Signals ......... 46
9. Troubleshooting for ActiGait® ... 49
10. Warranty......................................... 51
11. Expected lifetime ....................... 52
12. Cleaning, Storage and
Disposal........................................... 52
12.1 Cleaning............................................ 52
12.2 Storage............................................. 53
12.3 Disposal ............................................ 53
13. Available Articles ....................... 53
14. Technical Data.............................. 55
14.1 ActiGait® Control Unit.......................... 55
14.2 ActiGait® Belt Clip .............................. 56
14.3 ActiGait® Belt Loop ............................. 56
14.4 ActiGait® Body Clip............................ 57
14.5 ActiGait® Antenna .............................. 57
14.6 ActiGait® Antenna Fixture..................... 58
14.7 ActiGait® Heel Switch.......................... 58
14.8 ActiGait® Heel Sock ............................ 59
14.9 19.9 ActiGait® Charger Cable .............. 59
14.10 ActiGait® Charger ............................. 60
14.11 ActiGait® Implant .............................. 61
14.11.1 X-Ray Identication......................... 62
15.Classication................................ 62
16. List of symbols............................. 63
16.1 Ingress Protection............................... 65
17. Declaration of Conformity ....... 65