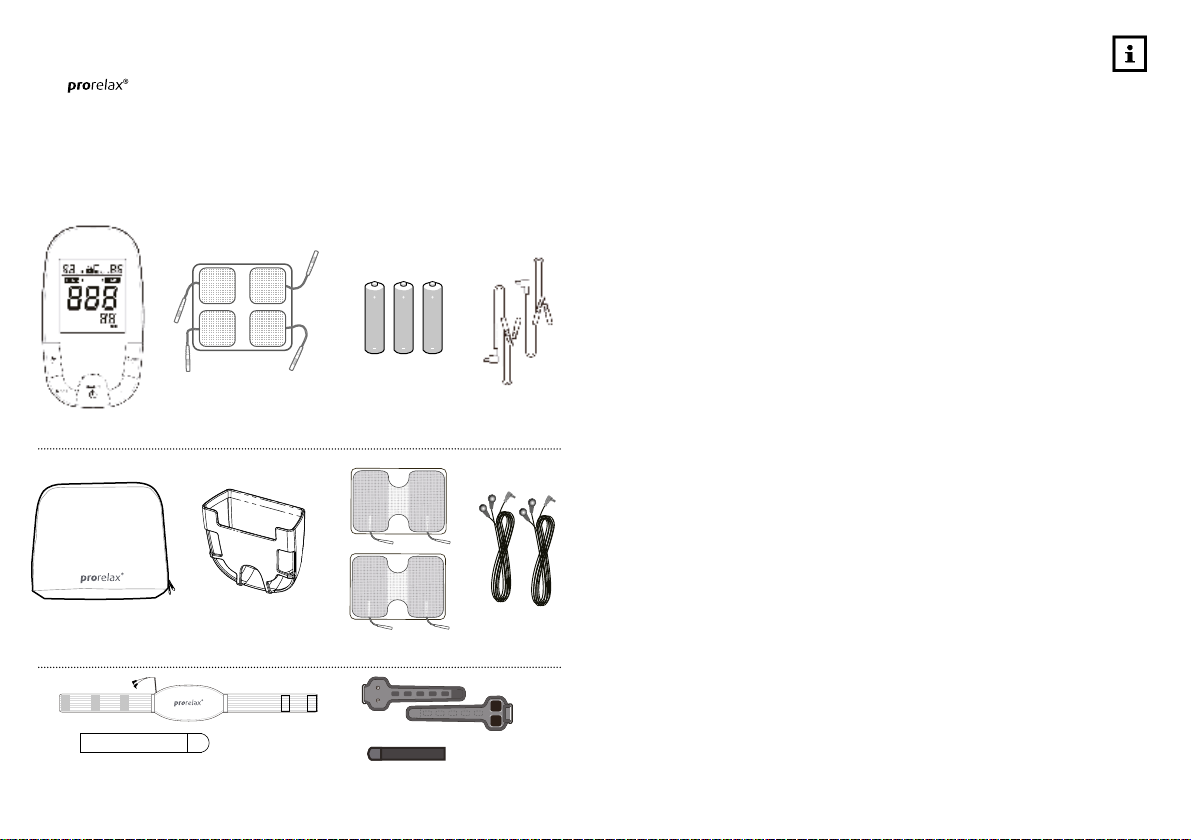
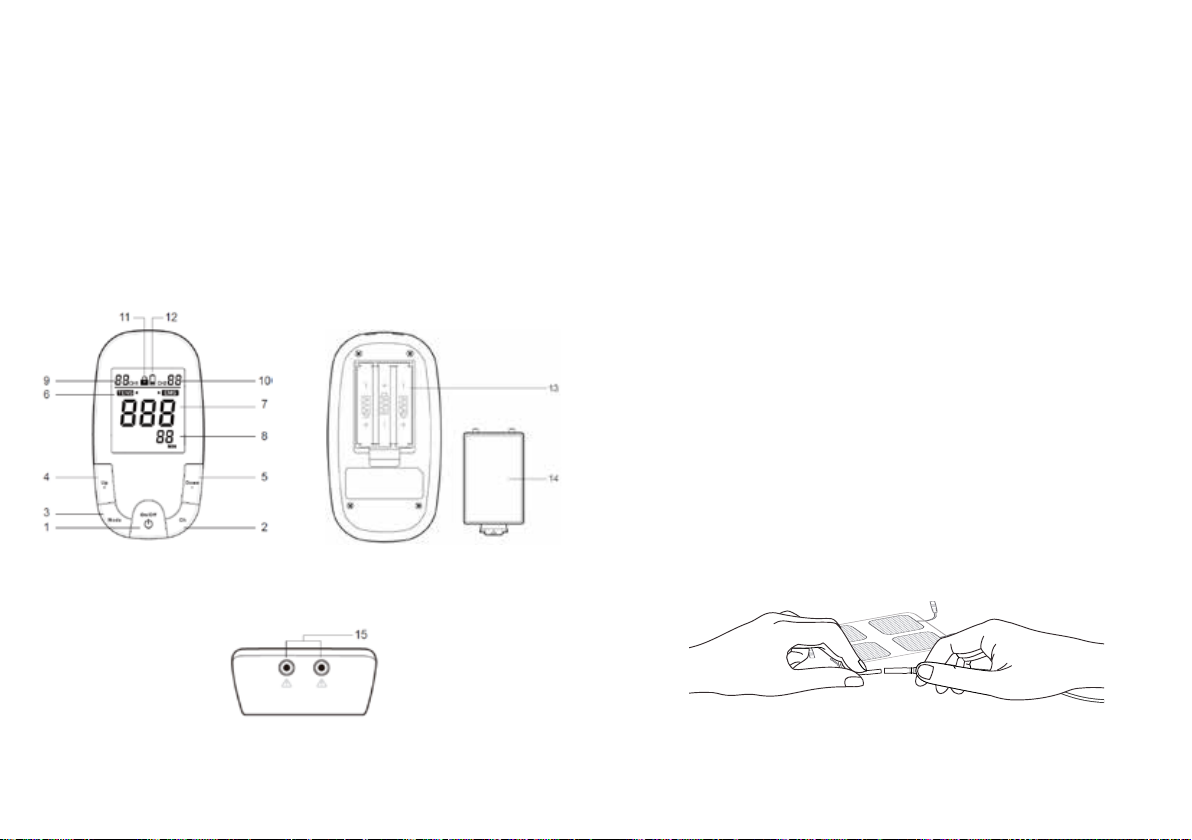
8 9
• The device must never be used near to the heart. The stimulation elec-
trodes must not be placed on any part of the front ribcage (where the
ribs and breastbone are located), especially not on the two large pecto-
rals. this can increase the risk of ventricular brillation and induce cardiac
arrest.
• On the skeletal skull structure, or around the mouth, throat or larynx
• In the neck/carotid artery area
• In the genital area
• On acutely or chronically diseased (injured or irritated) skin (e.g. inamed
skin – whether painful or not, reddened skin, rashes, e.g. allergies, burns,
bruises, swellings, both open and healing wounds, and post-operative
scars where the healing process could be aected)
• In humid environments (e.g. in the bathroom) / when bathing or showering
• Do not use after consuming alcohol
• If connected to a high-frequency surgical device
• In the case of acute or chronic diseases of the gastrointestinal tract
• The stimulation should not be applied above or through the head, direct-
ly onto the eyes, covering the mouth, to the front of the neck (especially
not to the carotid artery), or with the electrode surfaces placed on the
chest and upper back or across the heart.
Before using the device, consult your doctor if any of the following
applies to you:
• Serious illnesses, in particular if you suspect or have been diagnosed
with high blood pressure, a blood coagulation disorder, propensity to
thrombo-embolic conditions or recurrent malignant growths
• Any skin conditions
• Unexplained chronic pain in any part of the body
• Diabetes
• Any sensory impairment that reduces the feeling of pain (e.g. metabolic
disorders)
• If you are receiving medical treatment
• In the event of complaints linked to stimulation treatment
• If you suer from persistently irritated skin due to long-term stimulation
at the same electrode site
IMPORTANT!
Only use TENS+EMS SuperDuoPlus units:
• On adults
• For the intended purpose and as specied in these instructions for use.
Improper use can be dangerous.
• For external use
• With the original accessories supplied, which can be re-ordered. Failure
to do so invalidates the warranty.
PRECAUTIONS:
• Always pull rmly on electrodes to remove them from the skin to prevent
injuries in the unusual case of highly sensitive skin.
• Keep the device away from sources of heat and do not use it in close
proximity (approx. 1 m) to shortwave or microwave devices (e.g. mobile
phones), as doing so can result in unpleasant current peaks.
• Do not expose the device to direct sunlight or high temperatures.
• Protect the device from dust, dirt and humidity.
• Never immerse the device in water or other liquids.
• The device is suitable for self-treatment.
• For hygiene reasons, the electrodes may only be used on one person.
• If the device does not work properly, or you feel unwell or experience
pain, stop using it immediately.
• Switch o the unit or the respective channel rst before removing or re-
locating electrodes to prevent unintentional stimulation.
• Do not modify electrodes (e.g. by cutting them). This increases the
current intensity, which is potentially hazardous (max. recommended
output value for the electrodes is 9 mA/cm², an eective current intensity
beyond 2 mA/cm² requires increased awareness).
• Do not use the device whilst asleep, driving a vehicle or operating machi-
nery.
• Do not use whilst undertaking any activity where an unexpected reaction
(e.g. strong muscle contractions even at low intensity) could be dange-
rous.