Introduction
This guide contains specifications and guidelines for preparing your site for installation and operation of the Ionic™
Purification System. Prior to installation of your Ionic system, a PurigenBiosystems Field Service Engineer (FSE) will
contact you to schedule the install and confirm that your site meets the preparation requirements described in this
document. If your system installation has already been scheduled, and you believe your site may not be ready in
time, please contact your PurigenBiosystems Field Service Engineer (FSE) for assistance.
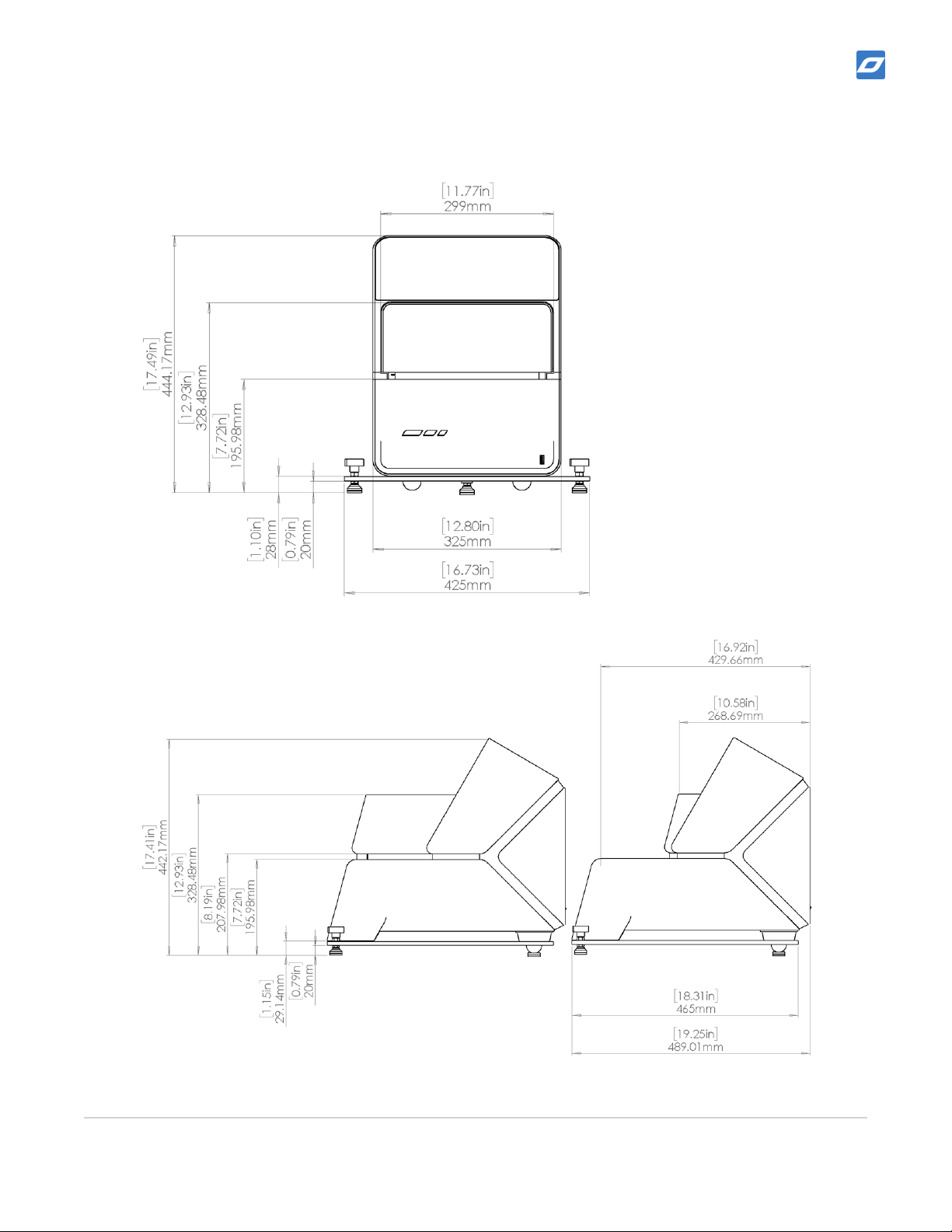
Laboratory space requirements
Electrical requirements
Environmental constraints
Computing requirements
User-supplied consumables and equipment
Additional Resources
The following documentation is available for download from the PurigenBiosystems website.
Guide Name Description
Ionic™ Purification System
User Guide
Provides an overview of instrument components and software, and procedures for proper instrument
maintenance and troubleshooting.
Ionic™ Cells to Pure DNA Kit Provides steps to use the Protocol and instructions for the Cells to Pure DNAKit.
Ionic™ FFPE to Pure DNAKit Provides steps to use the Protocol and instructions for FFPEto Pure DNA Kit.
Table 1: Additional Documentation Resources
Delivery and Installation
Following your purchase of an Ionic system, a PurigenBiosystems representative will contact you to confirm receipt
of your order and provide an early estimate of delivery. Prior to delivery, a PurigenBiosystems FSE will contact you
to schedule the installation.
The Ionic system will be shipped to you directly. In this case, you will receive shipment tracking information from
PurigenBiosystems. Inspect the packages containing your Ionic system and report any signs of damage or rough
handling to your PurigenBiosystems Support.
I M P O R T A N T Do not unpack the instrument prior to installation. The system must be
unpacked by a PurigenBiosystems FSE during installation.
C A U T I O N Only PurigenBiosystems personnel can unpack, install, or move the instrument.
Mishandling of the instrument can affect the alignment or damage instrument components.
DOCUMENT NUMBER:PUR-DOC-1
FOR RESEARCH USE ONLY AND NOT FOR USE IN DIAGNOSTIC PROCEDURES.5
IONIC™ PURIFICATION SYSTEM