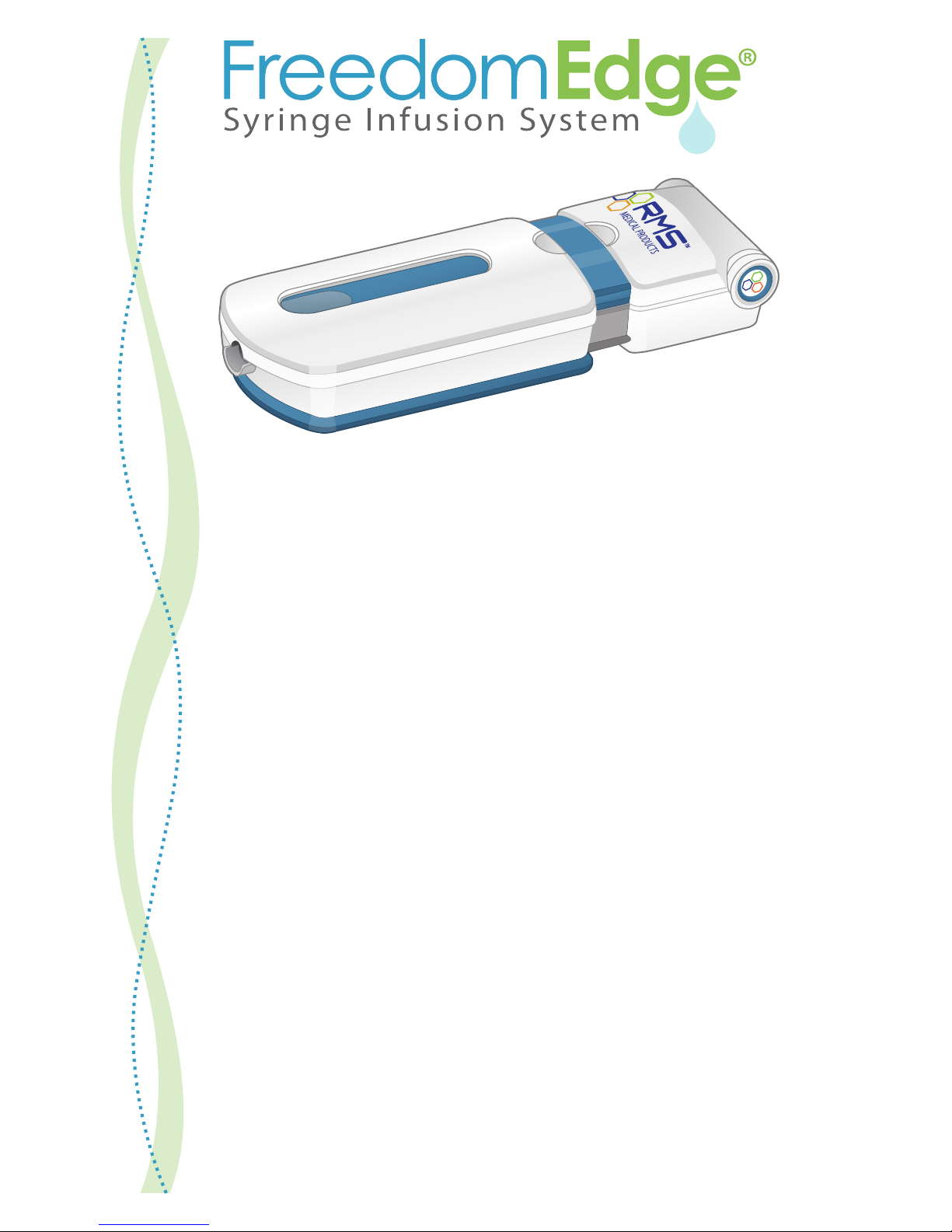
RMS Freedom Edge User manual
English 1 of 20
Introduction
Indications for Use
Caution
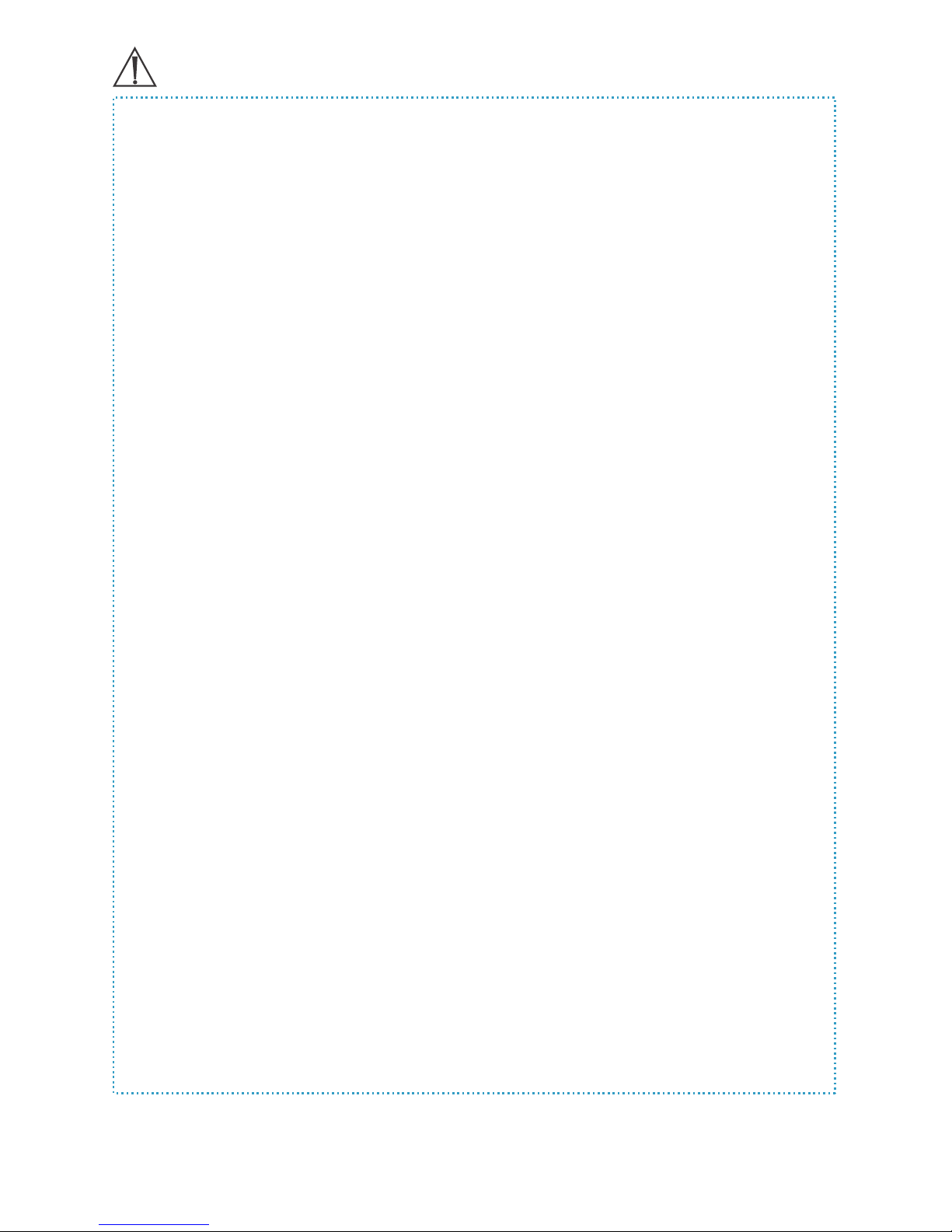
FreedomEdge® Syringe Driver Diagram
FreedomEdge® Product Listing
Syringes for Use with FreedomEdge®
Testing FreedomEdge® Syringe Driver
Syringe Loading and Removal Instructions
Selected Flow Rates vs Time
SubQ Infusion Instructions
Antibiotics Infusion Instructions
Troubleshooting
Care and Maintenance
Testing Accuracy
FreedomEdge® Flow Prole
Technical Specications
Selected Flow Rate Combinations
Warranty Information
Denition of Symbols
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . .
. . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2
2
3
4
4
5
5
6
7
7
9
11
12
13
14
14
15
17
18
Instructions For Use

English 2 of 19
Introduction:
The FreedomEdge® Syringe Infusion System is portable and easy to use, requiring
no batteries or electricity. It starts to operate when the syringe driver is closed. RMS
Precision Flow Rate Tubing™ sets are used to control the ow rate.
The FreedomEdge® operates at a constant safe pressure of 13.5psi. The constant
pressure developed in the FreedomEdge® automatically decreases the ow rate if
there is an increase in resistance during the delivery. The system will nd equilibrium
between the increasing resistance and ow rate. It provides constant ow which
tends to inhibit clots, and holds full pressure after an infusion is complete to prevent
blood or drug backow. The FreedomEdge® also eliminates concerns of a bolus,
overow, overdose, or runaway infusion.
The FreedomEdge® oers all the performance and technology of the FREEDOM60®,
in a design for 20ml and 30ml syringes. The FREEDOM60® is designed for
60ml syringes.
Indications for Use:
The Integrated Catch-Up Freedom Syringe Driver Infusion System (ICFSDIS), which
includes the FREEDOM60® and FreedomEdge® syringe drivers, is indicated for the
intravenous or subcutaneous infusion of medications and uids in the home, hospital,
or ambulatory settings when administered according to the approved biologic or drug
product labeling. The ICFSDIS is specically indicated for the subcutaneous infusion of
the following human plasma-derived immunoglobulins when used according to the
FDA approved biologic labeling: Hizentra, Immune Globulin Subcutaneous (Human)
20% Liquid (manufactured by CSL Behring); Gammagard Liquid, Immune Globulin
Infusion (Human) 10% (manufactured by Shire); and Cuvitru Immune Globulin Infusion
(Human) 20% (manufactured by Shire). The ICFSDIS is specically indicated for
the intravenous infusion of the following antibiotics when used according to the FDA
approved drug product labeling: meropenem, ertapenem, oxacillin, and tobramycin.
The FreedomEdge® Syringe Infusion System is indicated for use with the BD 20 ml (US
Reference number: 302830) or BD 30 ml (US Reference number: 302832) syringe. The
FREEDOM60® Syringe Infusion System is indicated for use with the BD 60 ml syringe
(US Reference number 309653).
English 3 of 20
Caution:
•Use the FreedomEdge® Syringe Infusion System only for the patient for whom the
device is prescribed and only for its intended use.
•
In order to achieve specic and repeatable ow rate performance with the Freedom
Syringe Infusion Systems’ unique constant force mechanism, use only Freedom System
accessories manufactured by RMS Medical Products. Directly connecting extension
tubing or administration set (without the luer disc) will cause the syringe to eject from
the syringe driver and eventually cause internal damage to it. Use of any other ow
control accessory may also cause over delivery of uids or medication to the patient.
For use with subcutaneous immune globulin products, use only RMS ow control
devices and HIgH-Flo needle sets, as use of generic products may result in unknown
ow rates and additional site complications such as pain, swelling and redness.
•Use only recommended syringes with the FreedomEdge®.
•Do not use syringes smaller than 20ml. The use of a smaller syringe may cause high
pressures that can be unsafe for the patient.
•Using the same tubing set, 30ml syringes will have slightly slower ow rates and slightly
increased delivery times versus 20ml syringes.
•Before use, carefully inspect the tubing set package. Do not use the tubing set if the
package is opened or damaged.
•Do not re-sterilize tubing sets.
•Overuse of the slide clamp or storing tubing sets with the slide clamp engaged for long
periods of time* may damage the tubing and aect the infusion rate.
•Carefully inspect the FreedomEdge® Syringe Driver before use. Verify its condition and
test. If the syringe driver is believed to not be operating properly or at the appropriate
rate of ow, immediately discontinue use.
•The FreedomEdge® Syringe Infusion System does not have an alarm, therefore no alarm
will sound if an interruption to ow occurs. There is no display of infusion status. The
syringe driver is not suitable for use with medication where delay or under-infusion
could result in serious injury.
•Discontinue use of a syringe driver that has been damaged, exposed to severe impact,
or which has failed to test properly.
•Discontinue use of a syringe driver that has been submerged in uid. If any uid is
allowed to enter the syringe driver, other than moisture from cleaning or sanitizing, it
should be replaced immediately.
•Do not autoclave the FreedomEdge®. It will melt the plastic and damage the syringe
driver.
•Federal law (USA) restricts this device to sale by or on the order of a physician.
•Tubing that is pre-primed and shipped at temperatures below freezing could
be damaged.
•The FreedomEdge® Syringe Infusion System is not intended for blood transfusions.
*Generally greater than 2 hours.
English 4 of
20
Product Listing:
Each FreedomEdge® Syringe Infusion System
includes a carrying pouch and user manual.
Product
Part #
FreedomEdge® Syringe Driver
F10020
FreedomEdge® Carrying Pouch
347400
DRIVER NOSE
PROGRESS
WINDOW closed
FRONT LIP
extended
FreedomEdge® Syringe Driver Diagram:
fully extended
and open
SYRINGE
PUSHER
ARMS
SYRINGE
LOCATOR
LUER
CRADLE
RMS Precision Flow Rate Tubing™ (case of 50)
SLIDE CLAMP
with PART #
END CAP
END CAP
LUER DISC
The part numbers for the RMS Precision Flow Rate Tubing™ represent the target ow rate
values for non-viscous solutions up to F120 (e.g. most antibiotics). For part numbers F180 and
higher, and all tubing for viscous solutions, the part numbers represent the median free ow
values, which are used in calculations to determine the actual ow rate delivered.
Part #Res. Vol.Part #Res. Vol.
F60
F120
F180
F275
F420
F500
F600
F900
F1200
F2400
0.14
0.16
0.13
0.11
0.10
0.09
0.09
0.08
0.13
0.15
F0.5
F1
F2
F3
F3.8
F5
F8
F10
F15
F30
F45
0.09
0.08
0.10
0.09
0.09
0.08
0.08
0.14
0.11
0.13
0.11

English 5 of 20
Syringes For Use with the FreedomEdge®:
•Becton Dickinson & Co. BD® Luer-Lok™ sterile 20ml (US Ref. number: 302830)
•Becton Dickinson & Co. BD® Luer-Lok™ sterile 30ml (US Ref. number: 302832)
1. Examine the inside to ensure it is free of debris or contamination.
2. Test to make sure the syringe locator moves freely by sliding it up
and down with your nger.
Note: For bench testing of ow accuracy refer to page 10. Note that the negator
mechanism which drives the syringe is calibrated for the life of the syringe driver. RMS
Precision Flow Rate Tubing™ sets are always measured to ow specication during
manufacture and will deliver the appropriate ow rate under controlled conditions.
Testing the FreedomEdge® Syringe Driver:
Part #: F10050
For Larger Volume Infusions:
The FreedomEdge® oers all the performance and technology of the FREEDOM60®,
in a design for 20ml and 30ml syringes. For larger volumes, the FREEDOM60®
accommodates 60ml syringes.
Step-by-step instructions including preparation, priming, checking for blood
return, infusing and concluding the infusion can be found on the FreedomEdge®
Sub-q Infusion Mat (03-4012). For use with antibiotics, refer to FreedomEdge®
Antibiotic Infusion Mat (03-1111).
English 6 of 19
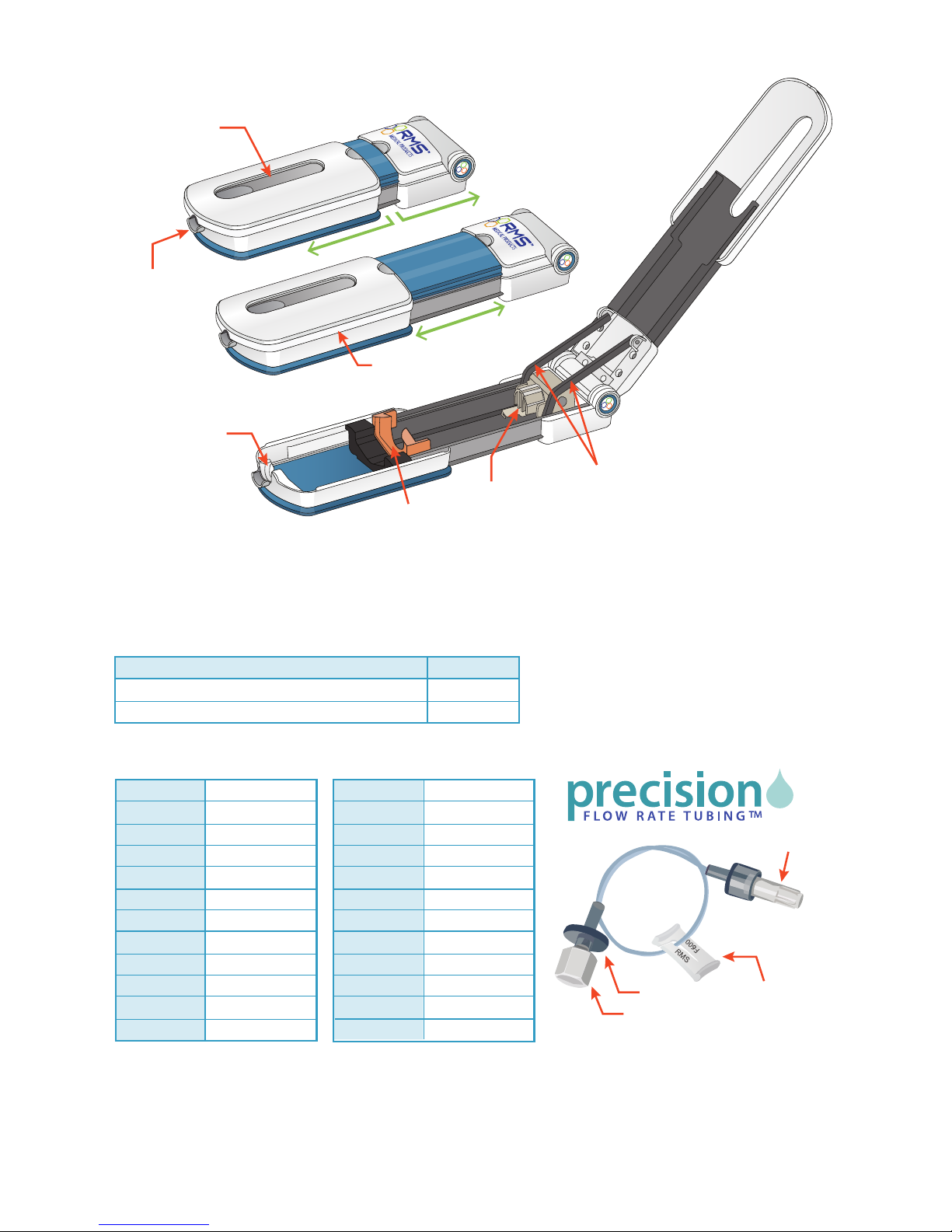
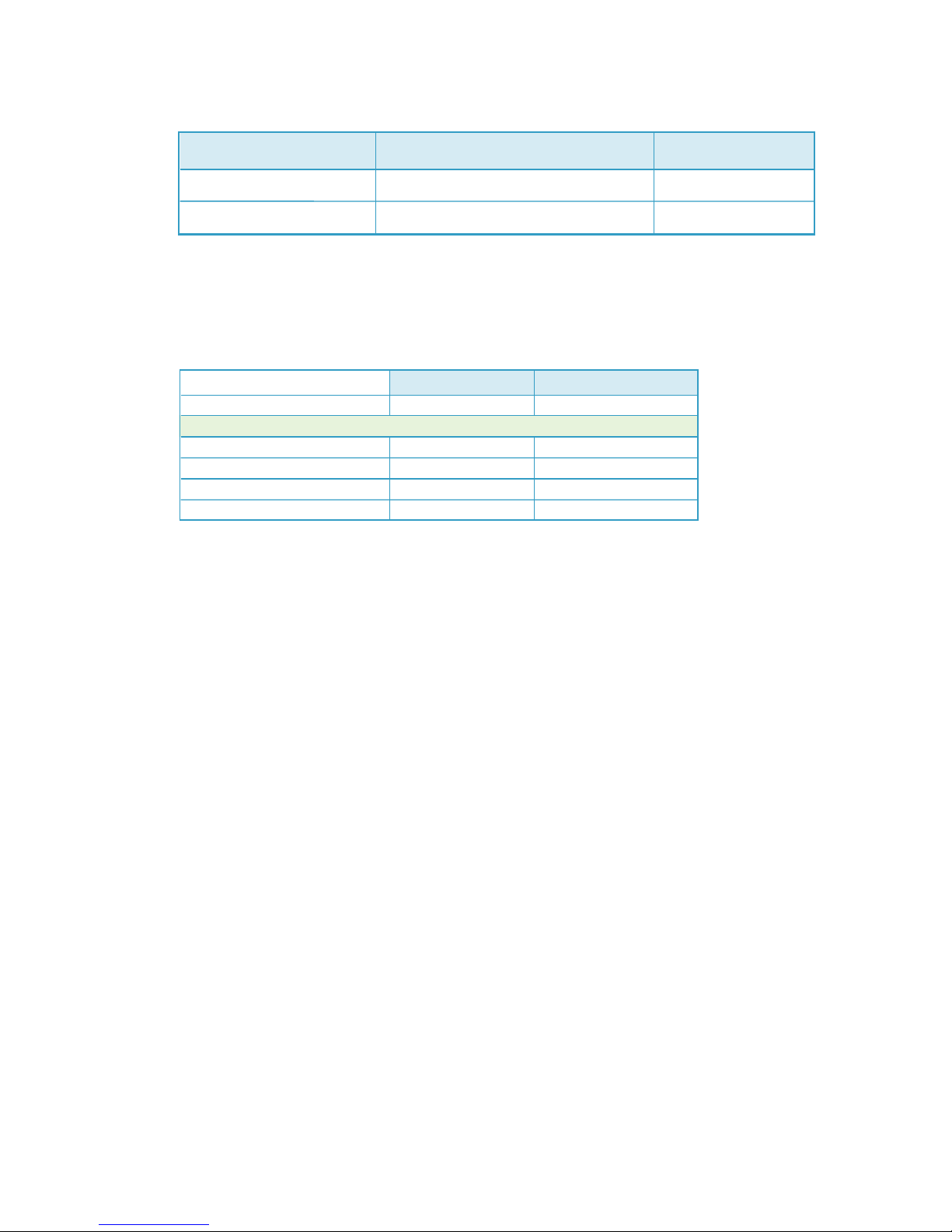
Syringe Loading and Removal Instructions:
To load a syringe:
To remove a syringe:
1. Pull rmly to fully extend the syringe driver.
2. Then, open the syringe driver fully by lifting the top cover.
3. With syringe gradations facing up, push the syringe against the syringe locater.
4. Seat the syringe ange into the syringe locater.
5. Seat the luer disc inside the syringe driver nose so that the syringe is rmly
attached inside the syringe driver.
Note: You can test proper t by gently tugging on the syringe. It will stay in place
if properly attached.
After the infusion is complete, open the syringe driver.
1. Remove the empty syringe by gently pushing it back to disengage the nose.
2. Lift the syringe up, out of the syringe driver.
Note: You will never need to use force to load or remove a syringe.
syringe ange
syringe
locator
English 7 of 20
Flow Rate vs Time Chart:
(suitable for subcutaneous and intravenous infusions)
1ml/hr 2ml/hr 30ml/hr 45ml/hr 60ml/hr 120ml/hr
5ml 5 hrs. 2 hrs. 30 min. 10 min. 6 min. 42 sec. 5 min. 2 min. 30 sec.
10ml 10 hrs. 5 hrs. 20 min. 13 min. 18 sec. 10 min. 5 min.
15ml 15 hrs. 7 hrs. 30 min. 30 min. 20 min. 15 min. 7 min. 30 sec.
20ml 20 hrs. 10 hrs. 40 min. 26 min. 42 sec. 20 min. 10 min.
25ml 25 hrs. 12 hrs. 30 min. 50 min. 33 min. 18 sec. 25 min. 12 min. 30 sec.
30ml 30 hrs. 15 hrs. 60 min. 40 min. 30 min. 15 min.
Syringe
Volume
Selected Flow Rates vs Time:
Note: For assistance in determining which RMS Precision Flow Rate Tubing™ set
to use, please contact RMS Medical Products at 800-624-9600.
Note: Tubing that is pre-primed and shipped at temperatures below freezing
could be damaged.
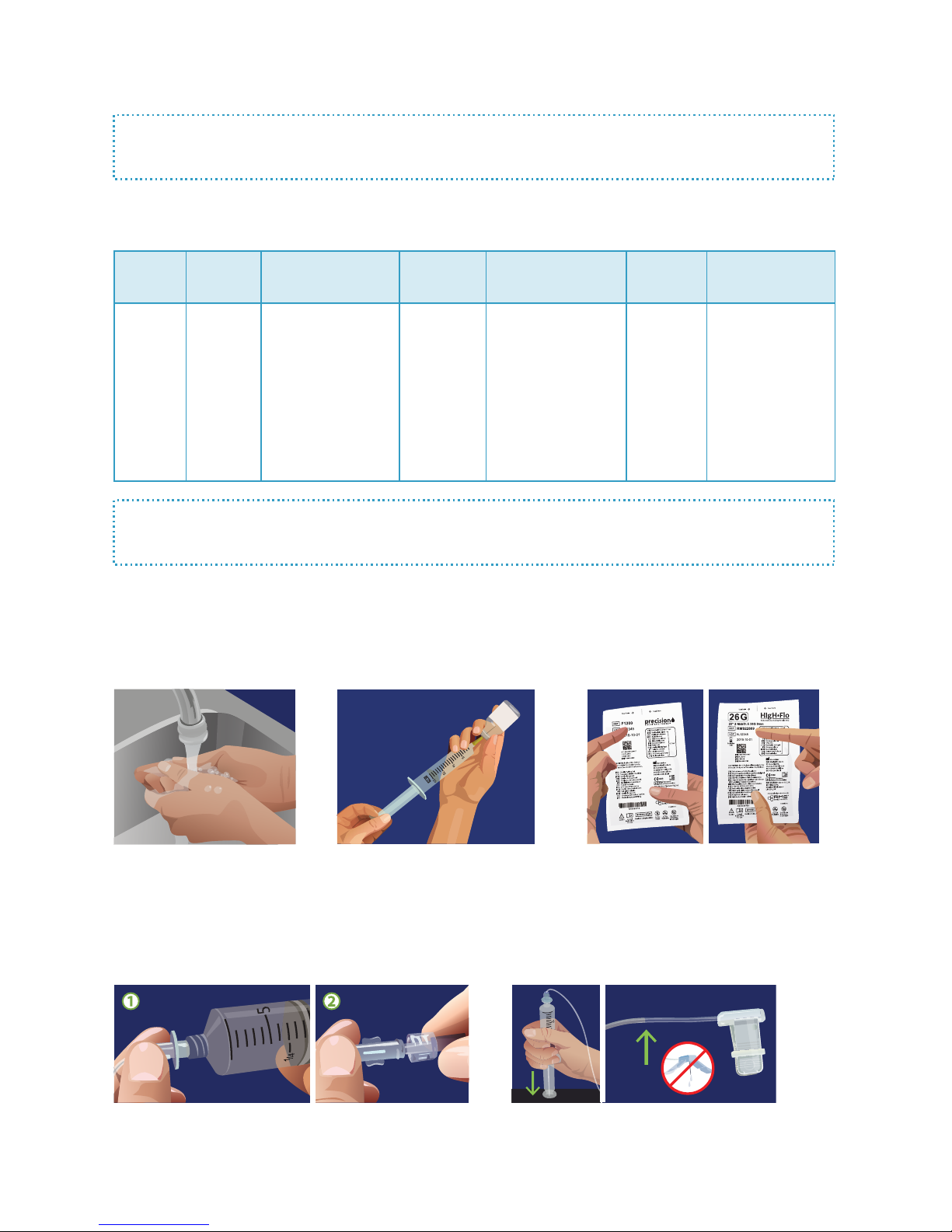
2 Fill Syringe
4 Attach Syringe, Tubing & Needles
1 Wash Hands
Prepare and Prime Tubing:
Verify that you are using the correct
tubing and needle sets recommended
by your healthcare provider and
prescribed by your doctor.
3 Verify Tubing & Needles
Wash your hands thoroughly
and, if required, put on
disposable gloves.
Be sure the product is at room
temperature before you begin
lling syringe with your required
dose. Refer to the manufacturer's
instructions or ask your provider for
more detailed lling instructions.
1. Remove the sterile cap from the luer disc end of the RMS
Precision tubing set and connect to syringe. 2. Remove
sterile caps from opposite end of tubing set and HIgH-Flo
needle set and connect, using care not to contaminate ends.
5 Prime Needle Set
Grasp the syringe, and gently press down with steady
pressure. Watch the tubing ll and stop ow about 2”
short of the needles. To minimize site irritation, it
is recommended to insert needles dry.
For SubQ Infusions, follow these instructions:
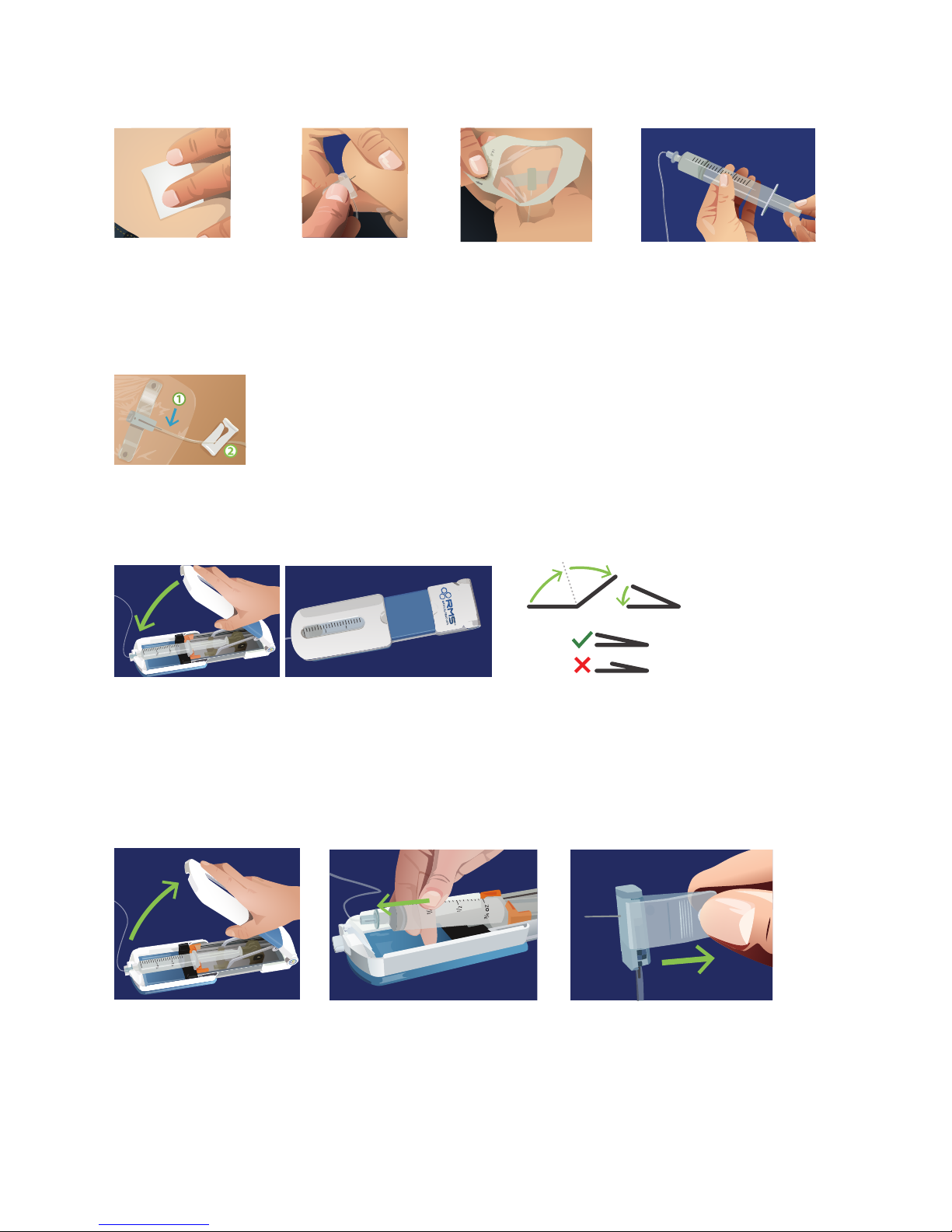
English 8 of 20
Select your site(s), cleanse
with alcohol and let dry.
Carefully remove shield
from the needle tip with-
out touching the needle.
1 Prepare Sites 2 Insert
Insert Needles/Check for Blood Return:
Pinch the skin and
insert each needle
into the subcutaneous
tissue at a 90˚ angle.
Remove the syringe from the
syringe driver. Check for blood
return by gently pulling back
on the syringe plunger.
3 Secure 4 Pull Back
Secure needle(s) with
adhesive dressing.
5 Watch
1.
Watch to make sure no red/pink appears in tubing near your sites.
2.
If blood return exists, clamp the ow to that site and call your healthcare provider to
determine if the dose can be run using the remaining sites. If so, continue. If not, remove
all needles, attach a new needle set and start again from step 5 of Prepare section.
1 Close Syringe Driver
Starting Infusion:
Start the infusion by closing the syringe driver. The infusion will begin immediately.
Note:
To pause infusion, simply open the syringe driver. To continue the infusion, re-close the
top lid. When closing the syringe driver, make sure that the top lid is fully extended and aligns
with the bottom portion.
2 Remove Syringe
Remove the empty syringe.
After Infusion is Complete:
(When syringe is completely empty)
One at a time, hold each needle in
place and peel back any adhesive/
dressing around it. Remove needle
in a straight motion. Remove needle
set from the syringe. Cleanse each
site and cover with a bandage.
Discard all sharps and biologics
as required.
1 Open Syringe Driver
3 Remove needles, discard sharps
Turn the syringe driver
OFF by opening the
top lid.
Hold needle in place and peel
back any adhesive/dressing
around it. Remove needle(s) in
a straight motion, opposite the
direction you inserted it

English 9 of 20
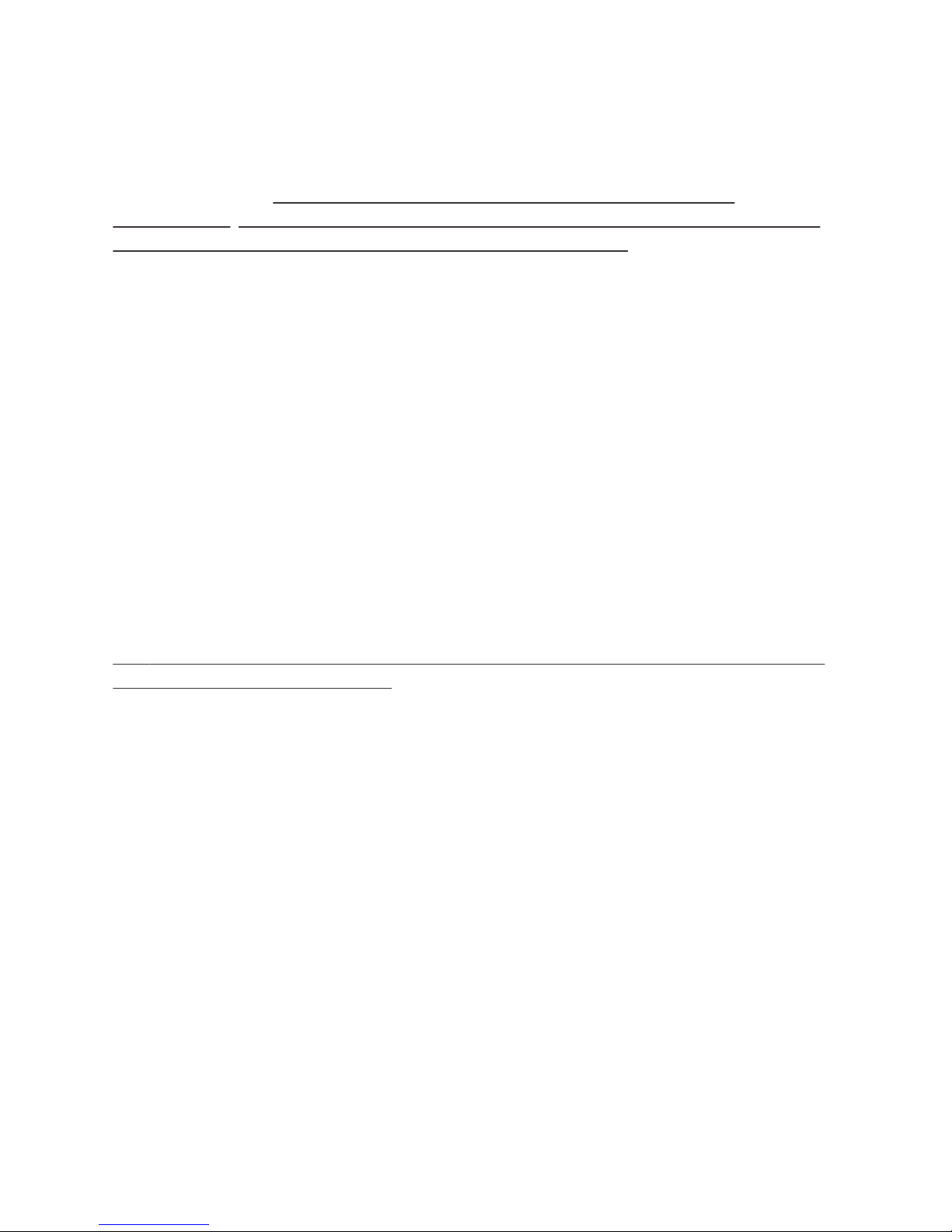
For Antibiotics Infusions, follow these instructions:
1 Wash Hands 3 Loosen Cap
4 Push Plunger 5 Watch for Drip & Tighten Cap
Prepare and Prime Tubing:
Attach the RMS Precision tubing
set to the syringe. Check the
F-number on the tag to make
sure you have the correct set.
2 Attach Tubing
Wash your hands thoroughly
and, if required, put on
disposable gloves.
Loosen the cap on the RMS
Precision tubing set.
When medication
starts to drip,
tighten the cap
on the tubing.
Grasp the
syringe, and
gently press
down with
steady pressure.
1 Clean Valve 2 Attach Flush
3 Open Clamp 4 Flush
Open the clamp
on the access
catheter and/or
extension set.
Follow your providers
instructions to dis-
infect your valve
using an alcohol pad
or disinfection cap.
Avoid touching the
disinfected opening
of the valve.
Flush with a
gentle push
and pause
method.
Flush:
Always follow your provider’s protocol.
Connect the
syringe directly
into the valve and
turn clockwise.

English 10 of 20
1 Connect 2 Open Syringe Driver
3 Load Medication Syringe 4 Close Syringe Driver
Infuse Dose:
Uncap the RMS Precision tubing set.
Turning clockwise, connect the
RMS Precision tubing to the valve.
Check that all clamps are open.
Close the syringe driver to begin
the infusion. Periodically check
the progress window to see that
the syinge driver is working, until
syringe is empty.
1. Load syringe into the syringe driver, plunger end rst. 2. If needed,
pull back on syringe locator to help 3. seat the luer disc into nose of
the syringe driver. The syringe should sit in front of the locator.
1. To open, conrm that the FreedomEdge® is fully extended.
2. Then, open syringe driver fully by lifting top cover.
1 Clamp 2 Open Syringe Driver
After Infusion is Complete: (When syringe is completely empty)
3 Detach Tubing
4 Flush & Clamp 5 Remove Syringe
Repeat Flush steps, and if in-
structed by nurse - ush with
Heparin. Clamp when done.
Disconnect the RMS Precision
tubing set and attach sterile
protection cap to set.
Clamp the access catheter
and/or extension set.
Turn the syringe driver OFF
by opening the top lid.
Open the syringe driver and
remove the syringe.

English 11 of
20
Note: If the dose is greater than 20ml/30ml and an additional syringe is required,
load the additional syringe by repeating section titled,‘Starting the Infusion’.
Troubleshooting:
If the suggestions in this section do not solve your problem, or
if problems persist, discontinue use and consult RMS Medical
Products and/or your healthcare provider.
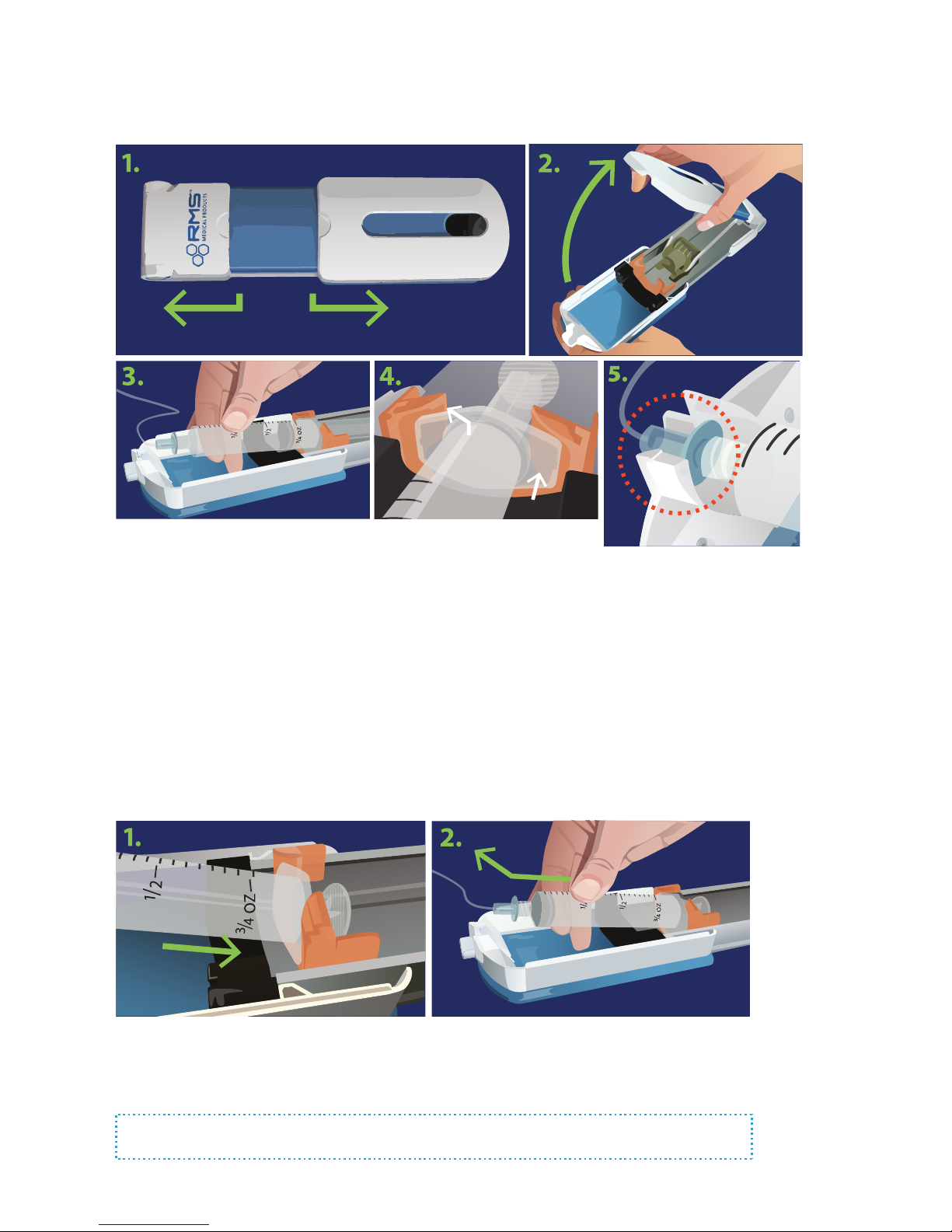
Syringe will not load or remove from syringe driver
Note: You will never need to use force to load or
remove a syringe.
1. Make sure the syringe driver is fully open, and that
nothing is blocking the syringe locator.
2. Confirm you are not filling the syringe to more than
30ml, or using a syringe larger than 30ml.
3. If you still have difficulty, use one hand to slide the
syringe locator all the way back, then place the syringe.
Syringe will not stay inside in the syringe driver
1. Make sure you are using the RMS Precision Flow Rate
Tubing™ sets with the luer disc and the RMS tag.
2. Make sure the luer disc end of the tubing has been
connected to an approved syringe and that the disc
is seated properly inside the nose of the syringe driver.
3. Make sure the flange shape of the syringe is correctly
seated into the shape of the syringe locator.
No flow
•Open and close the lid to ensure the syringe
pusher slides freely and does not bind.
•Make sure the slide clamp is unclamped and has
not been used for an extensive period of time. If the
slide clamp is overused it can damage the tubing.
•Test the tubing: Use sterile procedures to disconnect where the indwelling
needle or catheter is connected to the rate controlled tubing set; check for
medication drip. If medication does not drip, replace the tubing as it may be
blocked or damaged. If medication does drip from the RMS Precision Flow Rate
Tubing™, then it is most likely a problem with the indwelling needle, catheter,
or any fluid path accessories such as a clave connector or needle-free adapter.
Slow flow
•Verify that you are using the proper tubing.
• Verify that you are using the proper syringe. 30ml syringes will flow approx.
73% of the rate of a 20ml syringe (e.g. 120ml/hr tubing will flow at approx.
87.6ml/hr).
fully open
syringe ange
syringe
locator

English 12 of 20
Slow flow (continued)
•If a slide clamp is used for an extensive period of time, it can damage the tubing
and affect the flow rate. Try using another tubing set and measure the flow. For
60ml/hr tubing, the syringe plunger should move 10ml in 10 minutes (1ml/min).
For 120ml/hr tubing, the plunger should move 10ml in 5 minutes (2ml/min).
Stopping the flow quickly
•The syringe driver is designed to maintain pressure during and after the
infusion to prevent blood/drug backflow.
•To stop the flow, fully open the lid to relieve pressure from the syringe plunger.
•You can also use the slide clamp. Only use the slide clamp in the case of an
emergency, or when necessary to immediately stop the flow. Overuse can
damage the tubing.
Care and Maintenance:
The FreedomEdge®does not require any preventative maintenance. The
FreedomEdge®with RMS Precision Flow Rate Tubing™ works as a system, which means
the tubing determines the flow rate, not the syringe driver; therefore your driverneeds
no calibration. Selecting the appropriate tubing set for the application ensures the
proper flow rate will be achieved. During manufacture, RMS Precision Flow Rate
Tubing™ sets are thoroughly quality-tested to high standards, to guarantee flow rate
accuracy and appropriate flow rate delivery under controlled conditions.
Cleaning
Clean only those areas that are exposed and external. No attempt should be made
to clean any part of the syringe driver that is not easily accessible.
Wipe the outside surface with warm water and detergent or use any surface
disinfectant compatible with acetyl-butyl-styrene (ABS) plastic, such as hydrogen
peroxide. Avoid the use of alcohol or alcohol-containing compounds, as these tend
to make ABS plastic brittle. Wipe again with clean water to rinse.
If necessary, you may clean the inside of the syringe driver with a damp cloth and any
cleaning agent compatible with ABS plastic.
Storage
The FreedomEdge® syringe driver is recommended to be stored in a cool, dry place.
Packaged tubing sets should be stored at room temperature (approximately
16-30°C or 61-86°F).
Testing Flow Accuracy (if required by your local protocol)
1. Completely fill a new BD® 20ml syringe with sterile water. Do NOT use a 30ml
syringe for this test.
2. Remove all air from the syringe.
3. Attach a sterile RMS Precision Flow Rate Tubing™ set to the syringe.
4. Remove all air from the tubing set.
5. Load the syringe into the syringe driver and close the lid fully.
6. Monitor the syringe readings and elapsed time to derive an approximate
flow rate.
English 13 of 20
7a. Bench-Rated Flow Rate: The FreedomEdge® syringe driver design accounts for the effects
of standard clinical conditions on flow rate performance. Under bench test conditions, a 60ml/hr
labeled tubing set is designed to generate a nominal infusion rate of 72ml/hr. A 120ml/hr labeled
tubing set will generate a nominal bench test rate of 134ml/hr. The FreedomEdge® generates
nominal bench test rates higher than the labeled rate accounting for the following standardized
application criteria that affects actual delivery rates under normal clinical circumstances.
7b. Test Range: To assure consistent test results, keep syringe driver and tubing at the same
approximate horizontal plane and monitor flow until syringe empties (approximately 9 minutes
for F120 or 18 minutes for F60). The FreedomEdge® system is factory rated to deliver infusions
under strict test conditions over a large number of syringe drivers tested within 7% of nominal
with a 95% statistical confidence interval. Under varying bench test and fluid conditions this
range can be expected to vary approximately 15% of nominal. For more accurate monitoring, use
a stopwatch and finely graduated burette. FreedomEdge® syringe driver testing is based on
ANSI/AAMI National Standard, ID 26-1992, Infusion Devices, August 24, 1992.
Labeled Flow Rate Bench-Rated Flow Rate Test Range
F60 (60ml/hr)
72ml/hr
60-84ml/hr
F120 (120ml/hr)
134ml/hr
115-
15
3ml/hr
F60 (60ml/hr) Tubing F120 (120ml/hr) Tubing
Bench Test Rate
Less Clinical Eects
Catheter Gauge (20G PICC)
Fluid Viscosity
Venous Pressure
Label Flow Rate
72ml/hr
-7ml/hr
-2ml/hr
-3ml/hr
60ml/hr
134ml/hr
-8ml/hr
-3ml/hr
-3ml/hr
120ml/hr
If test results in the range indicated cannot be approximated under bench testing
conditions, contact RMS Medical Products at 1-800-624-9600.
References:
1. Stuhmeier, Mainzer B. MD; Aspects of pressure build-up in the use of electronic
infusion devices. II. Need for a pressure limit. Anasth Intensivther Notfallmed
(1987 Aug.) 22(4): 185-190.
2. Anasth Intensivther Notfallmed (1987 Aug.) 22(4): 181-184. ANSI/AAMI
National Standard, ID 26-1992, Infusion Devices, August 24, 1992.
Testing Flow Accuracy (continued)
7. Compare your test results to the range of test rates listed in the table below:

English 14 of 20
FreedomEdge® Flow Prole:
The FreedomEdge® flow profile shows that the flow rate is consistent throughout the
delivery of medication.
FreedomEdge®Flow Rate vs. Time
Fluid: H20 · Fluid Volume: 20ml · Tubing Measured: F120, nominal 133.55ml/hr
0.00
20.00
40.00
60.00
80.00
100.00
120.00
140.00
160.00
0 75 150 225 300 375
Flow Rate (mL/hr)
Time (seconds)
Technical Specifications:
System
Reservoir volume: 20ml/30ml
Residual volume: <0.4ml
Flow rate accuracy: ± 8%*
Operating pressure: 15psi (peak)/13.5psi (nominal)
(20ml reservoir)
Height sensitivity: ± 3% per 12” (30cm)
*Flow rate data recorded at 21°C using 20ml of 0.9% NS. An overall accuracy of ±8% is expected at
these values. At higher temperatures, which results in a decrease in viscosity, a higher flow rate is
anticipated to occur. The flow rate variation due to changes in temperature is approximately linear
and would vary from -20% at 14°C to +20% at 30°C. Fluids considerably more viscous than 0.9% NS
are expected to slow the flow rate and consequently may result in longer infusion times. More
viscous fluids may be tested before patient use by following the bench test procedures described
in this manual.

English 15 of 20
10 F120 RMS 1-2609 8.2 8.2 10 1:12 Suggested start Peds
10 F180 RMS 1-2609 10.5 10.5 10 0:57 Suggested start Peds
20 F275 RMS 2-2609 17.1 8.5 10 1:10 Suggested start Peds
20 F600 RMS 2-2609 29.6 14.8 10 0:40 Suggested start Peds
40 F600 RMS 3-2609 33.9 11.3 13.3 1:10 Suggested start Adult
40 F900 RMS 3-2609 44.3 14.8 13.3 0:54 Suggested start Adult
60 F900 RMS 4-2609 49 12.3 15 1:13 Suggested start Adult
50 F2400 RMS 3-2609 72.2 24.1 16.67 0:41
6
th
Infusion of biologic and beyond
100 F2400 RMS 4-2609 85.5 21.4 25 1:10 6
th
Infusion of biologic and beyond
(NEEDS TWO SYRINGES)
20 F600 RMS 2-2609 22.5 11.2 10 0:53 Suggested start Peds
30 F900 RMS 2-2609 28.3 14.2 15 1:03 Suggested start Adult
30 F2400 RMS 2-2609 41.9 20.9 15 0:42 6
th
Infusion of biologic and beyond
Hizentra – FreedomEdge® with 20ml ml syringe
Flow rate/site
(ml/hr)
Hizentra - with FreedomEdge® with 30ml syringe
Drug
volume (ml)
Flow Tube High Flo Set*
Flow Rate
Total (ml/hr) Time NOTES:
Vol/site
(ml)
Note 1.
Based on combining elements as written in Theory and Measurement of Fluid Flow
Rates in the Freedom system. Other combinations available per request.
Note 2.
24 G needles are not needed for performance up to 24.08 ml/hr for Hizentra.
Syringe Driver
Weight: 12oz (0.34kg)
Length: Closed: 9” (229mm)
Extended: 11.75“ (299mm)
Width: 3.25” (83mm)
Height: 1.5” (38mm)
Flow Rate Tubing
Length: 6” to 72” (152mm-1829mm)
Select Combinations of Flow Rates and Needles Sets for Use with
Hizentra, Cuvitru, and Gammagard Liquid
(Note that the following tables are only for the subcutaneous use of the immunoglobulin listed.)
RMS Precision Flow Rate Tubing™ / Residual Volume (ml)
Part #Res. Vol.Part #Res. Vol.
F60
F120
F180
F275
F420
F500
F600
F900
F1200
F2400
0.14
0.16
0.13
0.11
0.10
0.09
0.09
0.08
0.13
0.15
F0.5
F1
F2
F3
F3.8
F5
F8
F10
F15
F30
F45
0.09
0.08
0.10
0.09
0.09
0.08
0.08
0.14
0.11
0.13
0.11

English 16 of 20
**Indicates 24 gauge needle was used.
10 F275 RMS 1-2609 12.1 12.1 10 0:49 1
st
Two Infusions patients under 40kg
20 F275 RMS 1-2609 12.1 12.1 20 1:39 1st Two Infusions patients under 40kg
20 F600 RMS 2-2609 25.7 12.8 10 0:47
1
st
Two Infusions patients under 40kg
50 F600 RMS 2-2609 25.7 12.8 25 1:57 1
st
Two Infusions patients over 40kg
60 F1200 RMS 2-2609 37.1 18.6 30 1:37 Subsequent Infusions
60 F2400 RMS 2-2409** 110.5 55.4 30 0:32 Subsequent Infusions
60 F1200 RMS 1-2409** 55.3 55.3 60 1:05 Subsequent Infusions
100 F2400 RMS 4-2409** 132.8 33.2 25 0:45 Subsequent Infusions
20 F500 RMS 1-2609 12.9 12.9 20 1:32 1ST Two Infusions patients under 40kg
30 F900 RMS 2-2609 24.6 12.3 15 1:13
1
st
Two Infusions patients over 40kg
30 F2400 RMS 1-2609 21.2 21.2 30 1:24 Maintenance Infusions
30 F1200 RMS 1-2409** 42.1 42.1 30 0:42 Maintenance Infusions
Cuvitru – with FreedomEdge® with 30ml ml syringe
Cuvitru – with FreedomEdge® with 20ml ml syringe
Drug volume
(ml) Flow Tube High Flo Set* Flow Rate
Total (ml/hr)
Flow rate/site
(ml/hr)
Vol/site
(ml) Time NOTES:
(Note that the following tables are only for the subcutaneous use of the immunoglobulin listed.)
20 F45 RMS 1-2609 14.2 14.2 20 1:24 Patients under 40kg (Initial)
60 F120 RMS 2-2609 39.8 19.9 30 1:30 Patients over 40kg (Initial)
100 F420 RMS 4-2609 119.1 29.8 25 0:50 Patients over 40kg (maintenance infusions)
20 F120 RMS 2-2609 30 15 10 0:40 Patients under 40kg(Initial)
30 F180 RMS 2-2609 39.8 19.9 15 0:45 Patients over 40kg (Initial)
30 F120 RMS 1-2609 27 27 30 1:06 Patients over 40kg (Maintenance)
Gammagard Liquid – with FreedomEdge® with 30ml ml syringe
Gammagard Liquid – with FreedomEdge® with 20ml syringe
Drug volume
(ml) Flow Tube High Flo Set* Flow Rate
Total (ml/hr)
Flow rate/site
(ml/hr)
Vol/site
(ml) Time NOTES:
*Note:
HIgH-Flo needle sets: First number references the number of needles, the next two
numbers reference the needle gauge, and the last two numbers reference the needle length
in millimeters.
Warranty Information:
Limited Warranty:
RMS Medical Products/Repro Med Systems, Inc. (“Manufacturer”) warrants the
FreedomEdge® syringe driver to be free from defects in materials and workmanship
under normal use. Warranty is limited to Original Purchaser and covers the
FreedomEdge® for a period of two years from the purchase date. This warranty is not
valid for any damage caused by the use of non-RMS products. The “Original
Purchaser” is the person purchasing the syringe driver from the Manufacturer or
Manufacturer’s Representative. Warranty does not extend to subsequent purchasers.
Subject to the conditions of and upon compliance with the procedures set forth in this
limited warranty, the Manufacturer will repair or replace, at its option, any syringe
driver, or part thereof, which has been actually received by the Manufacturer or
Manufacturer’s Representative within the two-year warranty period, and which
examination discloses, to the Manufacturer’s satisfaction, that the product is defective.
Replacement product and parts are warranted only for the remaining portion of the
original two-year warranty period.
RMS rigorously tests the FreedomEdge® using RMS accessories to ensure that the
FreedomEdge® operates in accordance with published specification standards. If
non-RMS accessories are used in conjunction with the FreedomEdge®, RMS does not
represent that the FreedomEdge® will operate in accordance with published
specification standards. The FreedomEdge® warranty does not cover third-party
products or accessories.
The following conditions, procedures, and limitations apply to the Manufacturer’s
obligations under this warranty:
•Parties Covered by this Warranty: This warranty extends only to the Original
Purchaser of the syringe driver. This warranty does not extend to subsequent
purchasers.
• Warranty Performance Procedure: Notice of the defect must be made in
writing to Customer Support Department, RMS Medical Products/Repro-Med
Systems, Inc., 24 Carpenter Road, Chester, NY 10918, USA. Notice to RMS Medical
Products/Repro-Med Systems, Inc. must include the model and serial number, date
of purchase, and description of the defect in sucient detail to facilitate repairs.
Authorization must be obtained by the Original Purchaser from the Manufacturer
or Manufacturer’s Representative prior to returning the product to the
Manufacturer. The defective syringe driver must be properly packaged and
returned to the Manufacturer, postage-prepaid. Any loss or damage during
shipment is at the risk of the Original Purchaser.
• Conditions of Warranty: This warranty does not apply to any product, or part
thereof, which has been repaired or altered outside of the Manufacturer’s facility in
a way so as, in Manufacturer’s judgment, to aect its stability or reliability, or which
has been subjected to misuse, negligence or accident.
English 17 of 20

• Limitations and Exclusions: Repair or replacement of a syringe driver or
component part is the EXCLUSIVE remedy oered by the Manufacturer. The
following exclusions and limitations shall apply:
• No agent, representative, or employee of the Manufacturer has authority to
bind the Manufacturer to any representation or warranty, expressed or
implied, or to change this limited warranty in any way.
• THIS LIMITED WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES,
EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF
MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. THERE
ARE NO WARRANTIES THAT EXTEND BEYOND THE DESCRIPTION ON THE
FACE HEREOF.
• Manufacturer’s liability under this Limited Warranty Agreement shall not
extend to special, indirect, or consequential damages.
• The syringe driver can only be used under the supervision of medical
personnel whose skill and judgment determine the suitability of the
syringe driver for a particular medical treatment.
• All recommendations, information, and descriptive literature supplied by
the Manufacturer or its agents are believed to be accurate and reliable,
but do not constitute warranties.
This warranty and the rights and obligations hereunder, shall be construed under and
governed by the laws of the State of New York, USA.
Definition of symbols:
Manufacturer
Quantity
Caution
Catalog number
Consult instructions for use
Do not reuse
Authorized representative in the
European Community
Serial number
Use by YYYY-MM-DD or YYYY-MM
Sterilized using irradiation
Batch code
European Conformity
Do Not Use if Package
is Damaged Prescription Only
English 18 of 20

English 19 of 20

347200_Domestic_IFU_RevL
RMS Medical Products
24 Carpenter Road
Chester, NY 10918 USA
Ph. 800-624-9600 • 845-469-2042
www.rmsmedicalproducts.com
The FreedomEdge® Syringe Infusion System, FREEDOM60® Syringe Infusion System and RMS Precision Flow Rate
Tubing™ are registered trademarks of RMS Medical Products and are compliant with Medical Device Directive 93/42/EEC.
RMS Medical Products is ISO 13485 certied. © 2017 RMS Medical Products; All Rights Reserved.
Table of contents
Other RMS Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Blue 80/80 installation manual

Teesa
Teesa PX50 owner's manual

Cardiac Science
Cardiac Science Powerheart AED G3 Plus 9390A Quick reference guide

bort medical
bort medical MobiDig Extension quick guide

Solaire Medical
Solaire Medical Inner Space Ventaire Retrofit Installation

BodyMed
BodyMed ZZAN602 instruction manual