6
VIII–ANVÄNDA ENHETEN ................................................................................................ 37
IX –STÄNGA AV ENHETEN............................................................................................... 38
X –RUTINMÄSSIGT UNDERHÅLL/STERILISERING ..................................................................... 39
10.1 UNDERHÅLLNING AV IRRIGATIONSSLANG
.................................................. 40
10.2 UNDERHÅLL AV VINKELSTYCKE
................................................................ 40
10.3 UNDERHÅLL AV DET ROTERANDE INSTRUMENTET
....................................... 40
10.4 UNDERHÅLL AV ENHETEN
....................................................................... 40
10.5 MIKROMOTOR OCH SLADD
....................................................................... 40
XI –ÖVERVAKNING/FÖREBYGGANDE OCH KORRIGERANDE UNDERHÅLL ......................................... 41
11.1 ÖVERVAKNING
....................................................................................... 41
11.2 FÖREBYGGANDE OCH KORRIGERANDE UNDERHÅLL
.................................... 41
11.3 BYTE AV SÄKRING
.................................................................................. 42
11.4 OPERATIONSFEL
.................................................................................... 42
XII –ELEKTROMAGNETISK KOMPATIBILITET .......................................................................... 46
12.1 ELEKTROMAGNETISK EMISSION
................................................................ 46
12.2 ELEKTROMAGNETISK IMMUNITET
............................................................. 47
12.3 REKOMMENDERADE SEPARATIONSAVSTÅND
............................................... 48
XIII –AVFALLSHANTERING OCH ÅTERVINNING ....................................................................... 48
XIV –TILLVERKARENS ANSVAR.......................................................................................... 49
XV –BESTÄMMELSER ..................................................................................................... 49
XVI –SYMBOLER .......................................................................................................... 49
I - INTRODUCTION ....................................................................................................... 11
II –WARNINGS ............................................................................................................ 11
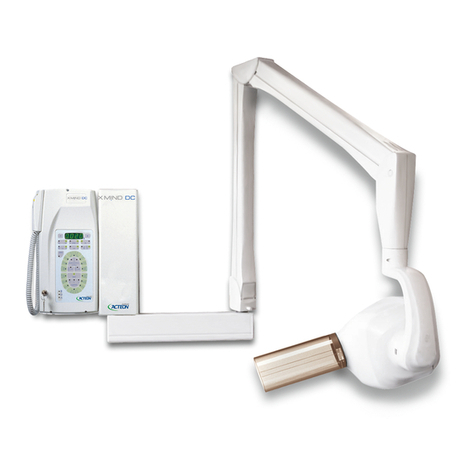
III –DESCRIPTION......................................................................................................... 14
3.1 PHYSICAL DESCRIPTION
............................................................................ 14
3.2 TECHNICAL DESCRIPTION
......................................................................... 14
a. LCD screen/control Keypad
.................................................................... 14