SmartLine RotaScope G Series User manual

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
1
© Copyright Smartline Medical Pty Ltd All rights reserved
RotaScope™
Vertical Hanging Scope Drying Cabinet
FEATURES:
•Endoscope capacity = 9 (suits most flexible scopes)
•X-ray or standard pass through options
•HEPA Filtered Air Drying - Independent air supply via Shared Air
Box (for up to 2 cabinets); or
•Remote Air Generator for multiple cabinets
•Vacuum channel drying reduces hook-up cross contamination
•Small footprint: 600mm(w) x 600mm(d) x 2335mm(h)
•Hygienic acrylic cabinet construction
•Interlocking RFID door locks
•Airflow, temperature, pressure and humidity monitoring and
reporting as per EN16442 Standards
•RFID Scope Data Monitoring and Display:
oCountdown timers for each scope.
oElectronic data backup and integration to other
software packages via CSV or MySQL.
oLive view of cabinets via software
•Data management system allows printing of labels to report
scope status, cabinet cleaning status, error status
•Discrete lighting indicates cabinet hygiene status and prompts
when cleaning or service is required
•Scope validation label with ‘Expiry Date and Time’ information
for Washer to Patient validation
•Stainless steel hooks and vacuum hose kits supplied (nine sets
including scope connectors)
•12 - 168 hours storage time –configurable

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
2
© Copyright Smartline Medical Pty Ltd All rights reserved
CONTENTS
CONTENTS...........................................................................................................................................................................2
NOTICE AND DECLARATION................................................................................................................................................6
PREFACE..............................................................................................................................................................................7
EN16442 COMPLIANCE REQUIREMENTS............................................................................................................................7
CABINET OVERVIEW ...........................................................................................................................................................8
Interlocking.......................................................................................................................................................................9
Storing Vertically ...............................................................................................................................................................9
AIR OPTIONS .....................................................................................................................................................................10
Benefits of Shared Air System..........................................................................................................................................11
Benefits of Vacuum Through Channels............................................................................................................................14
CABINET SPECIFICATIONS .................................................................................................................................................15
Cabinet Conditions.........................................................................................................................................................16
SpeCifications Table........................................................................................................................................................ 18
OPERATION INSTRUCTIONS..............................................................................................................................................23
Before Storage ...............................................................................................................................................................24
Hanging a Scope.............................................................................................................................................................24
Adjusting Top and Centre Disc Heights........................................................................................................................... 25
Connecting Vacuum to a Scope: .....................................................................................................................................26
DATA SYSTEM ................................................................................................................................................................... 28
Data System Overview................................................................................................................................................... 29
Count Down Timers ...................................................................................................................................................... 29
Scope and Staff Tags ....................................................................................................................................................... 30
Tag Programmer ................................................................................................................Error! Bookmark not defined.
Washer Station ...............................................................................................................................................................31
Sequence for Washing Scope: WHO, WHAT, WHERE ..................................................................................................32

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
3
© Copyright Smartline Medical Pty Ltd All rights reserved
Adding and Removing Scopes from the Cabinet ..............................................................................................................32
Printer Station.................................................................................................................................................................32
Replacing Labels ............................................................................................................................................................. 33
Laptop Kit....................................................................................................................................................................... 34
PC Operating Systems.................................................................................................................................................... 34
Software –Installed onto Laptop.....................................................................................................................................35
Positions of Components and Cable Distances................................................................................................................ 36
Closed Network –tcp/ip ................................................................................................................................................ 36
Cabinet Reset Button......................................................................................................................................................37
Printing Labels ................................................................................................................................................................38
INSTALLATION REQUIREMENTS........................................................................................................................................39
Information to Gather.....................................................................................................................................................40
Room Layouts, Ceiling Heights and Wall Construction ....................................................................................................40
Bulkhead ........................................................................................................................................................................ 42
Access and Route for Install.............................................................................................................................................43
Lifts / Stairwells ............................................................................................................................................................... 43
Install Dates, Work Hours...............................................................................................................................................43
Power Needs.................................................................................................................................................................43
STAFF TRAINING AND EDUCATION................................................................................................................................... 44
Standard Workflow......................................................................................................................................................... 45
Cabinet Reporting Conditions.........................................................................................................................................46
In-Service Training for Site .............................................................................................................................................. 47
Additional Training and Setup..........................................................................................................................................47
MAINTENANCE AND CLEANING........................................................................................................................................48
Vacuum Hoses –Cleaning IFU .......................................................................................................................................49
Cleaning ......................................................................................................................................................................... 49

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
4
© Copyright Smartline Medical Pty Ltd All rights reserved
Internal Surfaces .............................................................................................................................................................49
Exterior Surfaces.............................................................................................................................................................49
Cleaning Data Validation .................................................................................................................................................50
SAFETY .............................................................................................................................................................................. 51
Safe Operating Procedure...............................................................................................................................................51
Pre-Start Checks............................................................................................................................................................. 51
Turning On The Cabinet ................................................................................................................................................ 51
Maintenance Contracts ...................................................................................................................................................52
PQ –Performance Qualification......................................................................................................................................52
Compliance.................................................................................................................................................................... 53
TROUBLESHOOTING .........................................................................................................................................................54
PHYSICAL TESTS AND CHECKS...........................................................................................................................................56
Air Flow Check............................................................................................................................................................... 56
Endoscope Condition ..................................................................................................................................................... 56
Power Outages ..............................................................................................................................................................56
ANNEX A –VALIDATED SCOPES FOR STORAGE ................................................................................................................ 57
Validated Scopes Overview ............................................................................................................................................58
Olympus Colonoscopes .................................................................................................................................................58
Olympus Gastroscopes...................................................................................................................................................61
Olympus Duodenoscopes ..............................................................................................................................................62
Olympus Bronchoscopes................................................................................................................................................63
Pentax Scopes ................................................................................................................................................................64
Fujinon Scopes ...............................................................................................................................................................65
ANNEX B –INSTRUCTIONS FOR USE –ENDOSCOPE HOSE KIT CLEANING........................................................................67
Manual Cleaning .............................................................................................................................................................68
Batch Washers ...............................................................................................................................................................69

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
5
© Copyright Smartline Medical Pty Ltd All rights reserved
Sterilising for Sterile Storage ............................................................................................................................................ 69

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
6
© Copyright Smartline Medical Pty Ltd All rights reserved
NOTICE AND DECLARATION
This manual and any examples or images contained herein are provided “as is” and are subject to change without notice.
Smartline Machinery makes no warranty of any kind with regard to this manual, including, but not limited to, the implied
warranties of merchantability, non-infringement and fitness for a particular purpose.
Smartline Machinery shall not be liable for any errors or for consequential damages in connection with the furnishing,
performance, or use of this manual or the examples contained herein.
© 2017, Smartline Machinery
Product designed and manufactured by Smartline Machinery Pty. Ltd.
PO Box 532, Buderim, Qld 4556
Australia
Distributor Details:

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
7
© Copyright Smartline Medical Pty Ltd All rights reserved
PREFACE
Products made by Smartline Machinery Pty Ltd are designed to meet specific needs in the healthcare industry and we
manufacture in Australia under ISO 9001 quality system standards. Before our products leave the factory they undergo quality
assurance testing to ensure that they meet our quality standards. Should you experience problems with this product, please
contact us for support or repairs.
This User Manual covers the installation, general use and maintenance of the RotaScope™ Scope Drying Cabinet designed
and supplied by Smartline Machinery or approved distributors.
In countries outside Australia, our products are supported by local distributors which are always ready to help you. See
Distributor details on Page 5.
If the local distributor is unable to assist you, forward your support requests to Smartline Machinery at
tech@smartlinemachinery.com
This product has been designed to dry and store up to nine flexible Endoscopes. The construction and assembly is configured
to do so reliably and effectively in conjunction with European and Australian Standards and Guidelines.
Number of Scopes stored per cabinet: 9 Endoscopes. Capable of storing Gastroscopes, Colonoscopes, Bronchoscopes,
Duodenoscopes, EUS Scopes, Enteroscopes, ENT Scopes and more of most brands.
EN16442 COMPLIANCE REQUIREMENTS
Continuous monitored air-drying cabinets complying with European Standard EN 16442:2015 –for drying
Thermoliable Endoscopes. Globally this standard currently represents the highest documented level indicating best practice of
Scope storage at time of publication of this document.
EN16442 requires the cabinet to monitor, report and print various parameters including airflow status indication, cabinet
pressure, temperature, humidity, door open parameters and power outages.
All events are logged in the Data Management System for log retrieval and reporting
Maintenance: It is an operational requirement to have an up-to-date Maintenance Contract in place.
Validation: To maintain the accuracy of the environment monitoring equipment, the system needs to be recalibrated and
checked annually by an approved service agent. A Certificate of Compliance will be issued upon each annual recalibration. A
Validation Contract is separate to a Maintenance Contract, and is also an operational requirement.
RotaScope™ Cabinets meet current 2017 GENCA Guidelines and Position Statement March 2017 and are Compliant with
EN16442.
Australia: According to the GENCA Position Statement, March 2017, all cabinets must comply with EN16442 by December
2020.
This product is TGA listed –Article ARTG 140018. According to GESA/GENCA/ACIPC August 2017, “Statement 7a, “All
endoscopic instruments, except those in sterile packaging, should be stored in TGA-approved forced air drying cabinets”. And
Statement 7b, “Endoscopes stored in TGA-approved forced-air drying cabinets may be used for a period of up to 7 days
without reprocessing, unless otherwise stated by the manufacturer.

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
8
© Copyright Smartline Medical Pty Ltd All rights reserved
RotaScope™
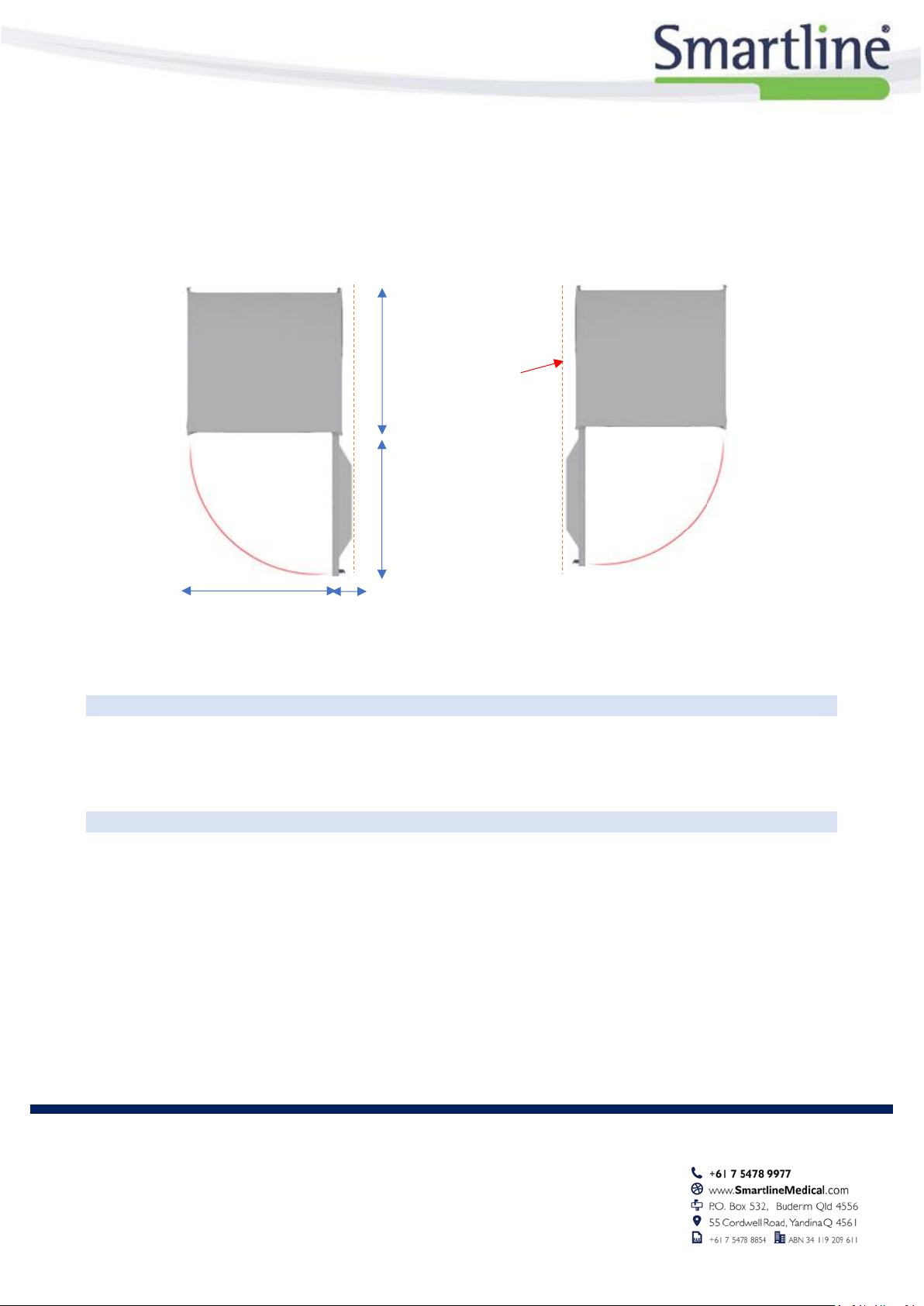
CABINET OVERVIEW
Acrylic Side Panels (white standard)
Clean Spot (inside door-
visible when open)
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
9
© Copyright Smartline Medical Pty Ltd All rights reserved
Door Opening Directions and Door Style Selection
RotaScope™ Cabinets come standard with doors hinged on the right. If there is a requirement that doors be hinged on the
left, this needs to be shown in the room layout drawings and be noted on all documentation. It is very important that this
information is detailed in the quoting stage, as although it is possible for the doors to be changed onsite, the client will incur
additional charges if changes were not requested in the original specification.
Right Hand Hinged (Standard)
Left Hand Hinged (upon request)
Right Hand Hinged (Standard)
Left Hand Hinged (upon request)
INTERLOCKING
If the selected RotaScope™ Cabinet is of the pass-through variety, the standard door locking mechanism will prevent both
doors from being open at the same time. This interlocking function will therefore prevent air from passing between the
cleaning and procedure rooms.
STORING VERTICALLY
By hanging scopes vertically, the detrimental memory effect produced by coiling scopes is reduced significantly.
600mm
600mm 80mm
565mm
Allow enough space
next to wall for door
with screen to fully
open
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
10
© Copyright Smartline Medical Pty Ltd All rights reserved
RotaScope™
AIR OPTIONS

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
11
© Copyright Smartline Medical Pty Ltd All rights reserved
BENEFITS OF SHARED AIR SYSTEM
The cabinet will provide an environment fit for drying qualified scopes stored inside.
Air flow into cabinet
Air flow out of cabinet
Air Flow Schematic

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
12
© Copyright Smartline Medical Pty Ltd All rights reserved
There are two options for the air generation for the RotaScope™ Cabinet. Both options are an inexpensive air supply and do
NOT connect to the hospital, medical grade, air supply. Low servicing costs of filters (annually) and pumps (bi-annually)
creates a cost-effective solution to airflow.
1. Remote Air System (Multiple Cabinets Use –Each site needs independent evaluation)
The Remote Air System can service 1 -7 cabinets and will require air ducting from the pump system (Remote Air Generator)
to the cabinets. The largest pump will require a 3-Phase power source.
2. Shared Air Box
The Shared Air Box can be positioned on top of a RotaScope™ Cabinet if the ceiling height is 2700mm. If the ceiling is lower
than this, it will have to be wall mounted using the SAB Wall Mounting Kit (09.SC-SB -WMK).
If a wall mount kit is used, there is a possibility that a 5-10 metre hose extension kit will be required to connect the SAB to the
RotaScope™ Cabinet. Similarly, a hose extension kit may be required to connect air to the secondary RotaScope™ Cabinet if
positioned up to 10m away from the primary cabinet that holds the SAB.
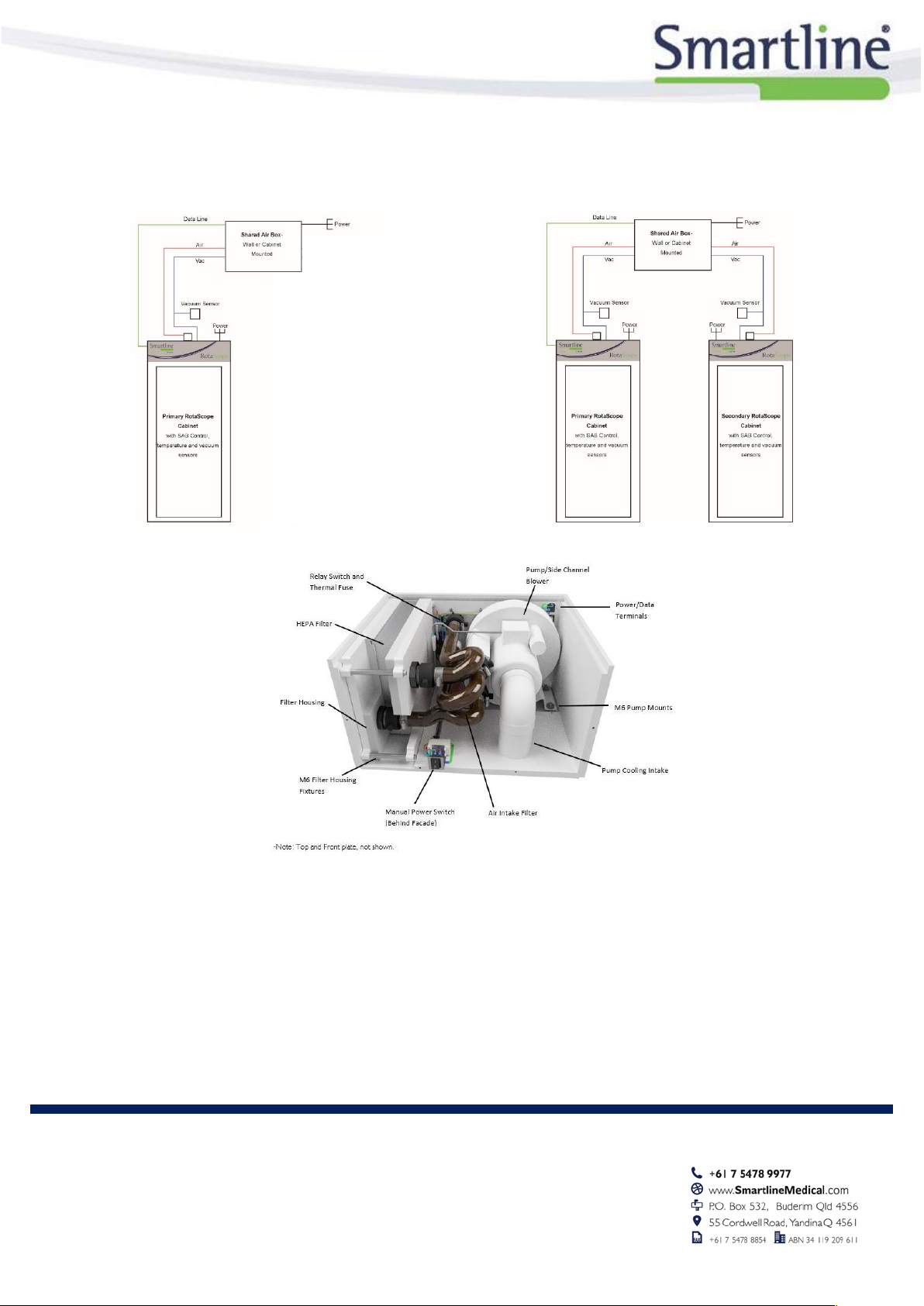
Shared Air Box
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
13
© Copyright Smartline Medical Pty Ltd All rights reserved
Single cabinet install schematic
Double cabinet install schematic
The Shared Air Box (09.SAB) will generate HEPA Filtered air and vacuum for up to two cabinets.
The Shared Air Box is located with the cabinets (no plumbing or construction work required)
The ongoing costs of this product is low, and it only requires basic servicing.

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
14
© Copyright Smartline Medical Pty Ltd All rights reserved
PUMP REPLACEMENT FILTER REPLACEMENT
The following table details replacement parts:
Year
1 Cabinet
2 Cabinets
3 Cabinets
4 Cabinets
5 Cabinets
1
1x
1x
2x
2x
3x Filters
2
1x
1x
2x
2x
3x
3
1x
1x
2x
2x
3x
4
1x
1x
2x
2x
3x
5
1x
1x
2x
2x
3x
1
N/A
N/A
N/A
N/A
N/A
2
1x
1x
2x
2x
3x Pumps
3
N/A
N/A
N/A
N/A
N/A
4
1x
1x
2x
2x
3x
5
N/A
N/A
N/A
N/A
N/A
BENEFITS OF VACUUM THROUGH CHANNELS
The RotaScope™ Cabinet uses vacuum which ensures enough airflow passes through each endoscope channel to assist with
drying the internal surfaces. The clean HEPA Filtered air is passed into the cabinet and across each scope, the air is pulled
through the distil tip of the scope and returns through the various hook-up manifolds.
QUANTITY OF CABINETS (One system can service two cabinets)

MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
15
© Copyright Smartline Medical Pty Ltd All rights reserved
RotaScope™
CABINET SPECIFICATIONS
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
16
© Copyright Smartline Medical Pty Ltd All rights reserved
CABINET CONDITIONS
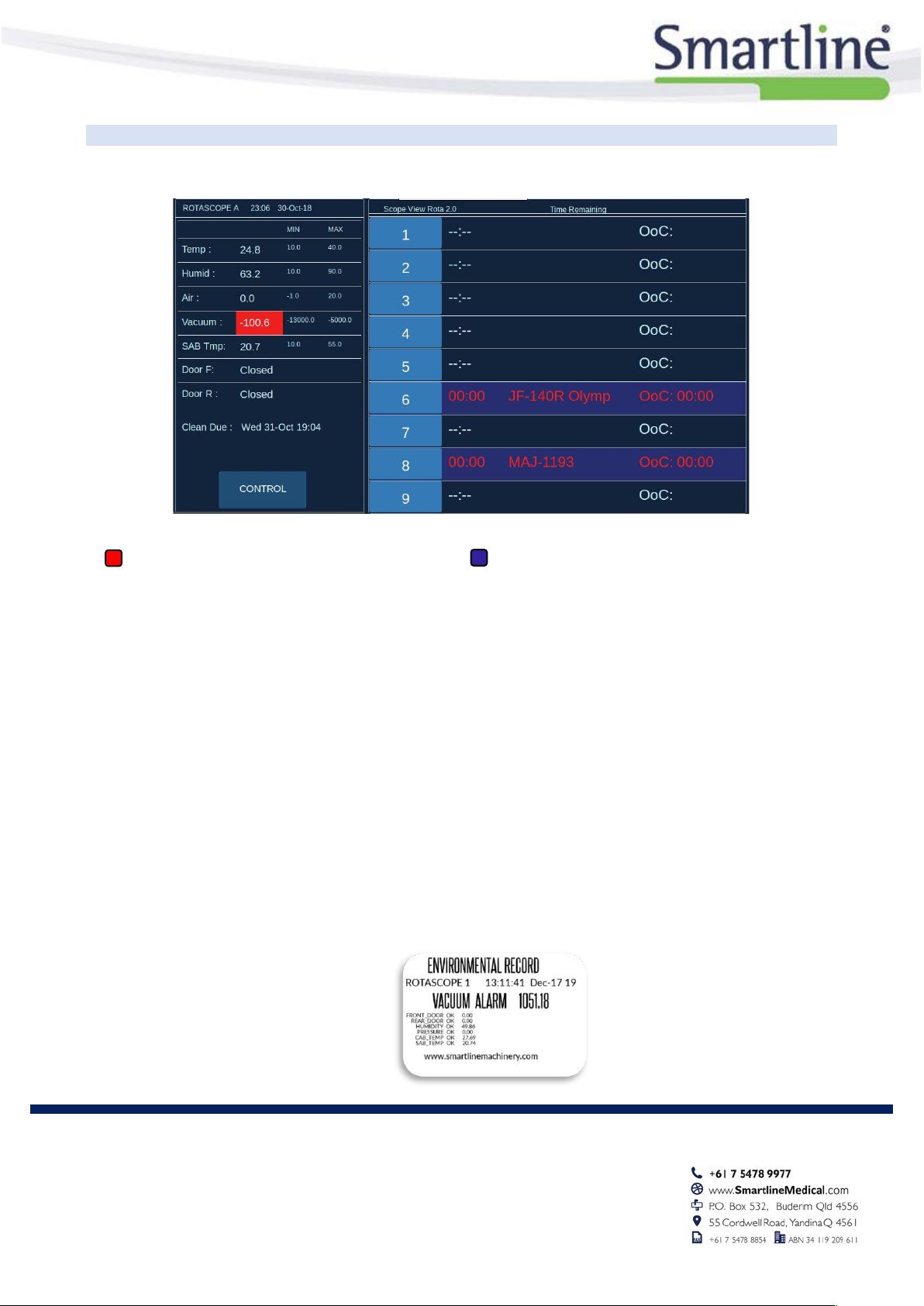
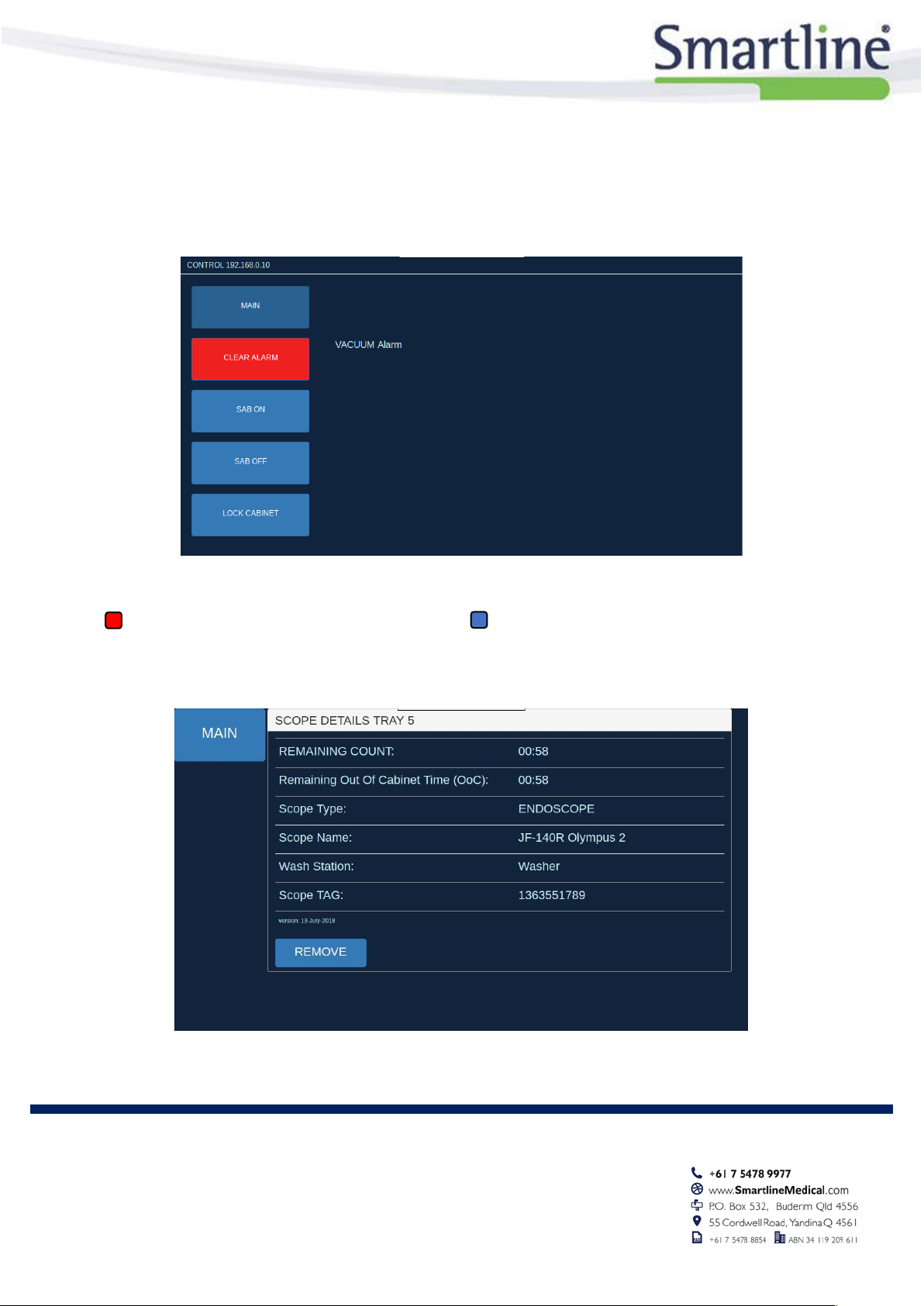
Cabinet Mounted Touch Screen Interface
Sensor Status Key
= Value out of range
= Scope Storage details (Expired in RED)
The RotaScope™ Environmental Monitor measures and records the following cabinet conditions:
•Temperature
•Humidity
•Air (Cabinet Pressure)
•Vacuum
•Door Status
•Shared Air Box Temp
•Routine Cleaning Status –Clean Due
•CONTROL –SAB and Alarm Status
•Scope Location Button –Detailed Storage Info
The air system automatically maintains a positive pressure within each cabinet. Having a positively pressured cabinet,
minimises external airflow (cleaning room / theatre air) into the cabinet. All the Environmental Conditions are measured and
recorded by the Data Management System as required to meet EN16442 Standards. If a deviant reading level is received (in
the Warning or Alarm values of these conditions), the following will occur:
•An automatically generated printout identifies actions for Cabinet Clean Completed and Alarms
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
17
© Copyright Smartline Medical Pty Ltd All rights reserved
•Cabinet lighting will change colour (each fault has an assigned colour, see: Warnings and Alarms section)
Cabinet Mounted Touch Screen Control Screen
Sensor Status Key
= Alarm Acknowledge Button
= SAB on/off
Cabinet Mounted Touch Scope Storage Details Screen
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
18
© Copyright Smartline Medical Pty Ltd All rights reserved
SPECIFICATIONS TABLE
Topic
Answer
Notes
List of endoscopes that can be stored in
the storage cabinet;
See: Approved Scope List.
Endoscope accessories (e.g. valves) can be
stored inside the storage cabinet but it is not
the intention of the storage cabinet to
maintain the microbiological quality of these
accessories.
Storage temperature band
10 –40degC
Maximum storage time
168hrs
7 days maximum storage
Drying function
N/A
Cabinet is not equipped with a drying function
Drying temperature band
N/A
Not Applicable as cabinet does not use
elevated temperatures to dry load.
Time required to dry the endoscopes (if
applicable)
3 Hours (MAX)
Dryness may be achieved in less than 3 hours
Channel aeration
Tested at IQ, OQ and PQ Intervals (Install
and Annual PQ)
Testing with use of Smartline Medical Aeration
Testing Device inline between scope channel
and vacuum manifold to check air circulation
through different channels.
Manifold Test with Oxyview
Automated channel flushing control
N/A
Automated channel flushing NOT used
Air Flow circulation diagram
See: USER Manuals
See: Benefits of Shared Air System
RotaScope:Page 10
SlidaScope: Page 10
Cabinet Flow Rate (Ports at Manifold)
>1.0 l/m
Minimum detectable flow rate is .5 l/m
Cabinet Flow Rate (Total)
200 l/m
10x Air Exchanges p/h
Minimum detectable flow rate is 20 l/m
Total Vacuum Flow Rate Per Cabinet
170l/m
Cabinet Pressure Band
-1–27pa
Differential Pressure (above 27Pa is possible
but not recorded.
Endoscopes that one or more channels
is below the detection limit
N/A
Currently there are no endoscopes that can be
stored in the cabinet that have a flow rate that
is below the detectable flow rate of 1 l/min.
Air Quality
99.99% @0.3µm Filtration Rate (HEPA
Filtered Supply Via SAB)
Third party air is not used by the storage
cabinet. A Shared Air Box draws required air
from the surrounding environment of which it
is situated and therefore standard office
working conditions need to be provided at all
times. Room temperature range 10 C° to 30 C°,
relative humidity between 40% and 70%,
environmental room pressure greater than 102
kPa.
Humidity Band
3.0 –90.0 %RH
Oil Content
N/A
Compressed air not used
Air Quality Testing Frequency
Annually –Service and PQ
HEPA Filter replaced Annually
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
19
© Copyright Smartline Medical Pty Ltd All rights reserved
Cabinet Cleanliness Level
N/A
A specific cleanliness level is not claimed –
Clinical Clean at commissioning/handover is
required by HSO required
Cabinet Cleaning Limits
<168hrs
Weekly cleaning required for cabinet and
accessories (Vacuum Hose Kits)
Cabinet Cleaning IFU
See: User Manual
See: Maintenance and Cleaning
RotaScope: Page 47
SlidaScope: Page 48
Accessory Cleaning IFU
See: Vacuum Hose Kit IFU Document
Microbiological Alert Levels - Airborne
See: PQ Contract
<50 CFU Acceptable
>50CFU = Failure
>1CFU must be identified
>1CFU Identified as Pathogenic = Failure
Testing carried out as part of Annual PQ in
accordance with AS4187 and EN16442
Microbiological Alert Levels - Surfaces
See: PQ Contract
<25 CFU Acceptable
>25CFU = Failure
>1CFU must be identified
>1CFU Identified as Pathogenic = Failure
Testing carried out as part of Annual PQ in
accordance with AS4187 and EN16442
Preventative Maintenance
See: Service Agreement and/or User
Manual
See: PQ –Performance Qualification
RotaScope: Page 52
SlidaScope: Page 51
Annual Preventative Maintenance of Cabinets
and Shared Air Systems are Recommended –
To be done by a Smartline Medical Accredited
Service Technician
Selectable storage cabinet cycles
Out of Cabinet Time –3hrs
Non-alcohol Cycle –12hrs
Alcohol Cycle –168hrs
See: Staff Training Manual
See: Wash Validation of Scopes
Transferring Endoscopes
See: User Manual
See: Storing/Hanging A Scope
RotaScope: Page 22
SlidaScope: Page 22
IFU for Power Outage
See: User Manual
After a power outage a cabinet will illuminate
purple and a label will be printed with
associated information.
See: Cabinet Reporting Conditions & Power
Outages
RotaScope: Page 46 & 54
SlidaScope: Page 45 & 53
Model Numbers and Serial Numbers
See: Cabinet
See: Compliance Page 53
Environmental Conditions
Standard office working conditions need to be
provided at all times. Room temperature range
10 C° to 30 C°, relative humidity between 40%
and 70%,
MAN-0901 RotaScope
VALID DATE: 01/2019 to 12/2020
Model: RotaScope G & Upgrades
20
© Copyright Smartline Medical Pty Ltd All rights reserved
Correcting, resetting and acting on
faults
See: User Manual
See: Cabinet Conditions
RotaScope: Page 16
SlidaScope: Page 16
Over and under pressure stabilisation
band
Instant
Cabinet Air pressure requires no time for
stabilisation
SAB Over Temp,
Instant
Alarm:
•Subtracts time from OoC
•Causes SAB to Shutdown
Self-rectifying –attempts to run again after 2
minutes.
Note: Alarm Acknowledge required at Cabinet
interface for normal operating function (Yellow
Cabinet Lighting) however, OoC will only count
down whilst the pump is not running.
Cabinet Temperature, Humidity, Air
Pressure, Vacuum and Door Alarms
3 Minutes
Warning Mode:
Yellow warning light for first three minutes
Alarm:
•Subtracts time from OoC
Self-rectifying Alarm, once cabinet reads in
normal status, the cabinet will return to green
and OoC will cease counting down.
Clean Alarm
24hr Warning (Blue Light)
Alarm:
•Subtracts time from OoC
Requires Cabinet Clean Function to rectify
alarm
Storage Expiry
Instant
Visible:
•00:00 On Door Screen
•“Re-Process Scope” on Label
OoC Expiry
Instant
Visible:
•00:00 On Door Screen
“Re-Process Scope” on Label
Endoscope Channel Condition Prior to
Storage
See: Automated Endoscope Reprocessor
(AER) IFU or Manual Cleaning Regime
The verification that all channels allow the
passage of air before the device is loaded into
the storage cabinet is required when a scope
has been cleaned and disinfected using a
manual cleaning procedure. For each cabinet,
one endoscope is to undergo an aeration test
(bubble test) as per manufactures instructions
during annual Performance Qualification. In
the case where the endoscope is cleaned and
disinfected using a validated processing
procedure (washers compliant with EN ISO
15883-4:2009) this verification is included
through channel monitoring however,
extensive air flushing of all channels is
recommended before placing the endoscope in
Table of contents
Other SmartLine Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Syneron
Syneron Candela VelaShape III System user manual

Creative
Creative PC-60N Instructions for use
Exsurco Medical
Exsurco Medical Amalgatome MD Quick reference guide

Topcon
Topcon RM-8900 instruction manual
Tecno-gaz
Tecno-gaz ANDROMEDA VACUUM xp user manual

Flight Medical Innovations
Flight Medical Innovations FLIGHT 50 Operator's manual

Boston Scientific
Boston Scientific LUX-Dx manual
GE
GE Centiva/5 Plus Technical reference manual

Stryker
Stryker Power-PRO IT Cot 6516 Maintenance manual

promotal
promotal iQuest 3050 Series user guide

Maxtec
Maxtec SmartStack Assembly instructions/Instructions for use

Otto Bock
Otto Bock 743L500 3D L.A.S.A.R. Posture Instructions for use






