steute RF 2PW-MED 2G User manual
Other steute Medical Equipment manuals

steute
steute KF 1PW-MED GP11 User manual
steute
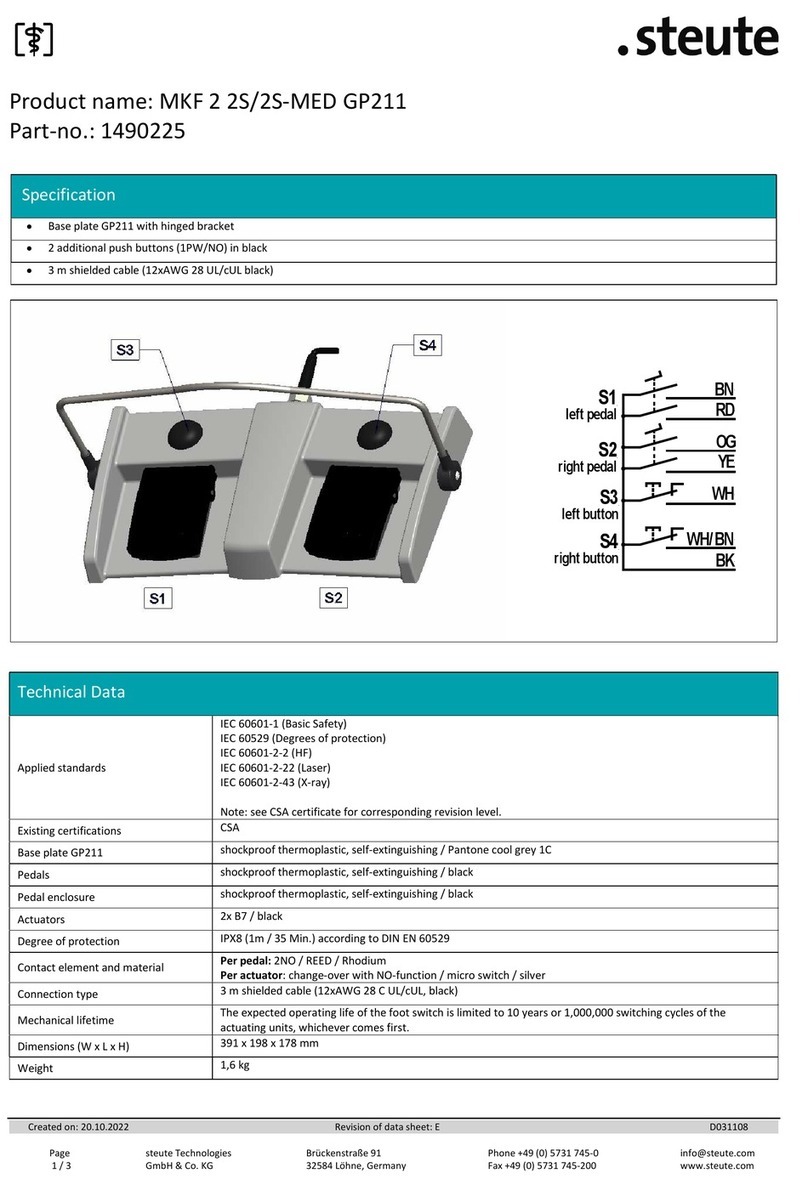
steute MKF 2 2S/2S-MED GP211 User manual

steute
steute MKF 3 2S SW2.4LE-MED GP311 User manual

steute
steute MKF 2 2S/2S SW2.4LE-MED GP212 User manual

steute
steute MKF 2PW-MED User manual

steute
steute MKF 2 2S User manual
steute
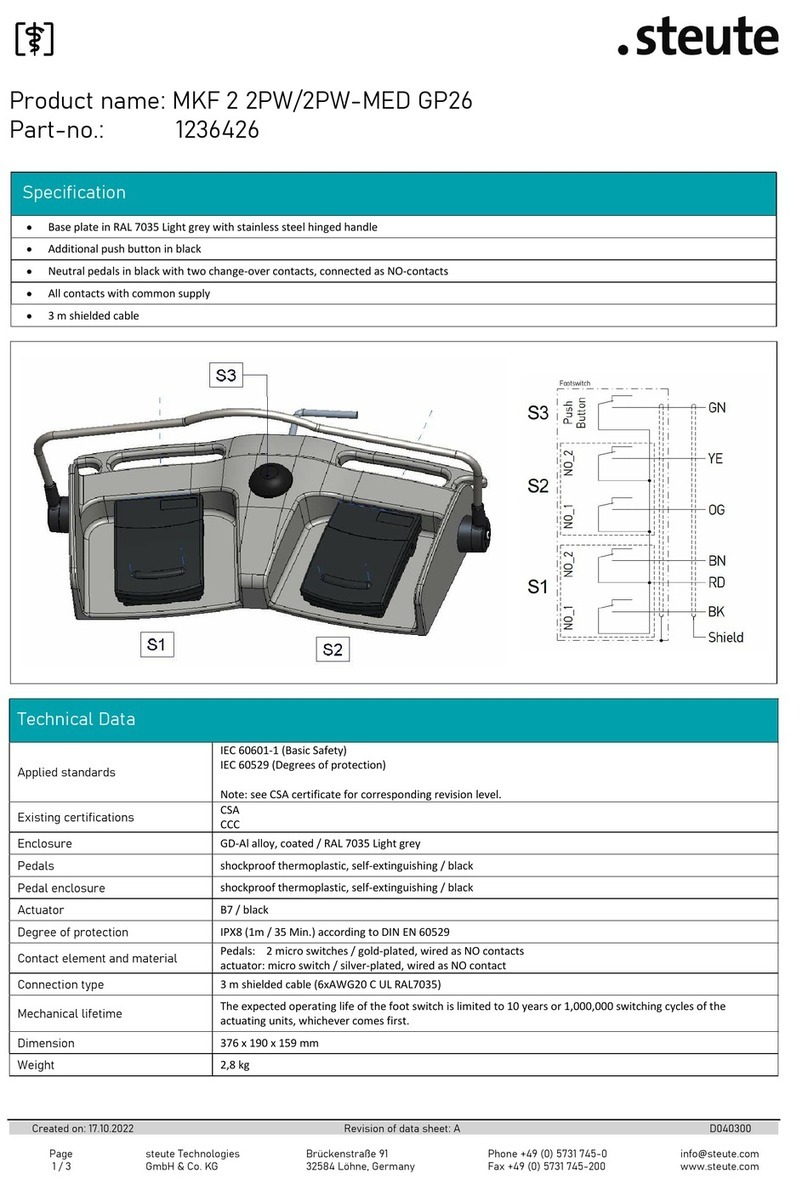
steute MKF 2 2PW/2PW-MED GP26 User manual
steute
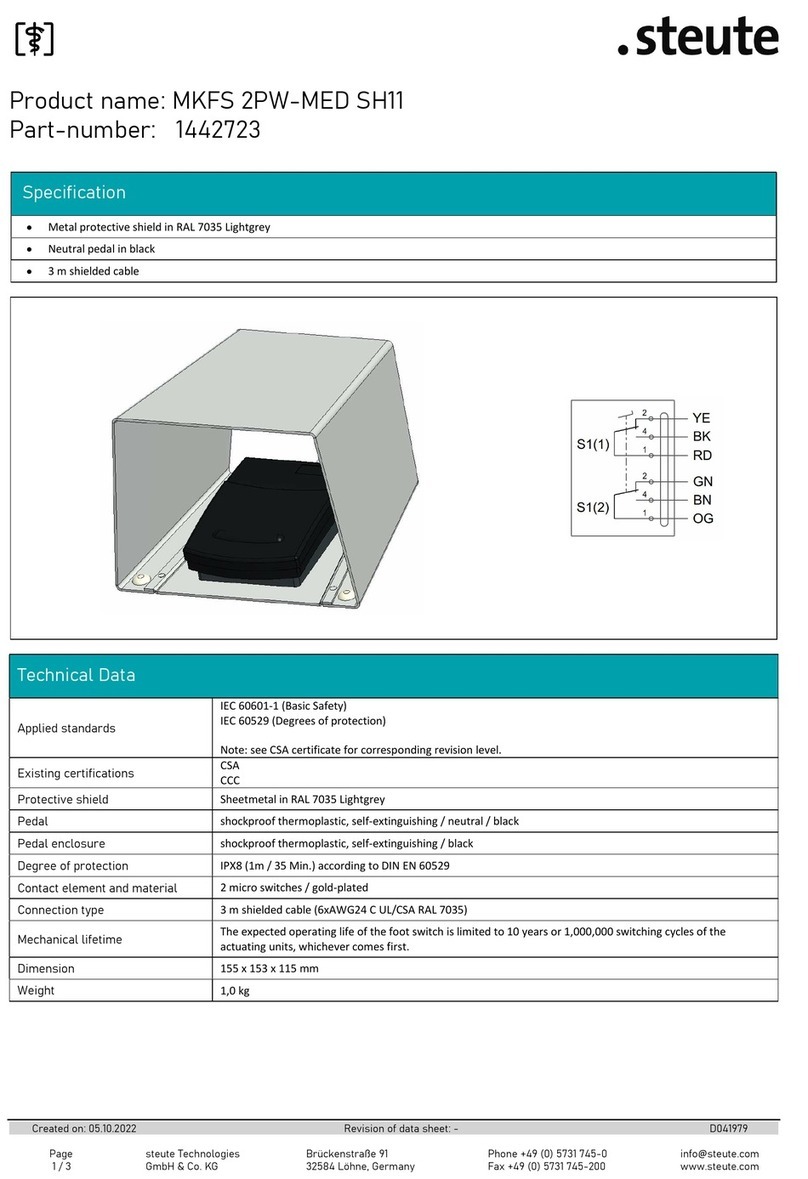
steute MKFS 2PW-MED SH11 User manual
Popular Medical Equipment manuals by other brands
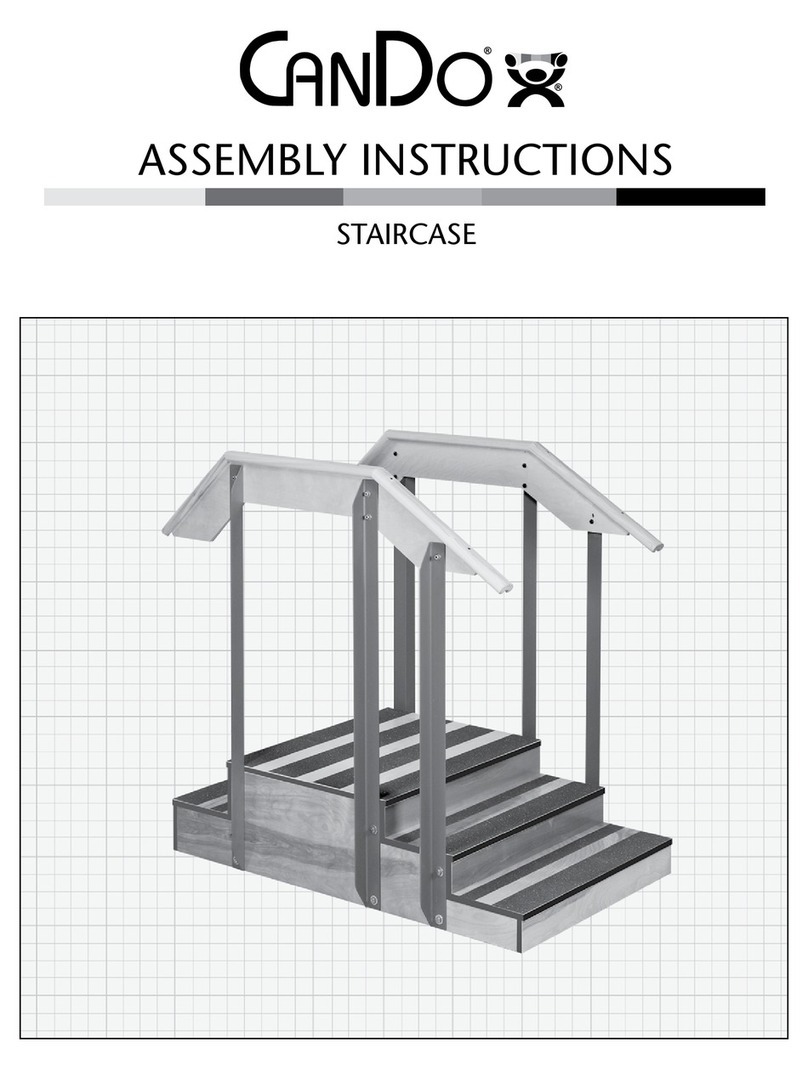
Cando
Cando STAIRCASE Assembly instructions

bort medical
bort medical 054 600 SP quick guide

Tecno-gaz
Tecno-gaz SLIM Instruction manual for use and installation
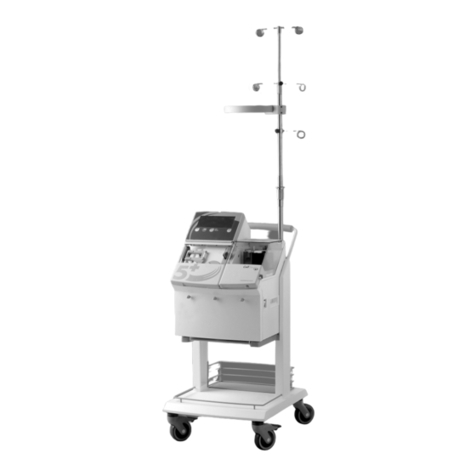
Haemonetics
Haemonetics Cell Saver 5+ Operator's manual

Clinical Health Services
Clinical Health Services TENS 3N1R Instructions for use

Aspect
Aspect BIS VISTA Service information manual
ZOLL
ZOLL ICY IC-3893A Instructions for use

baxter
baxter DUPLOSPRAY Quick reference manual
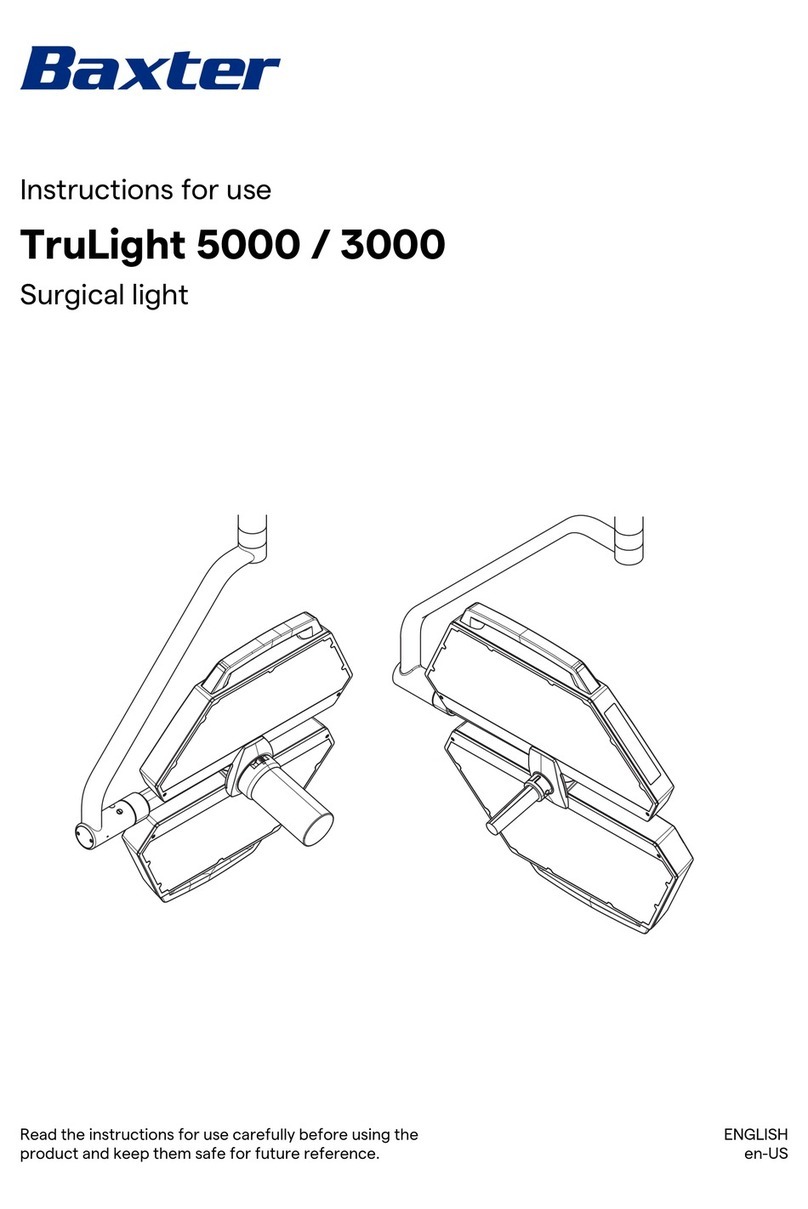
baxter
baxter TruLight 5000 Instructions for use

Fresenius Medical Care
Fresenius Medical Care DIASAFE 2008 K Operator's manual

OSCILLA
OSCILLA TSM-300 Instructions for use

Sissel
Sissel Cold Therapy Compression operating manual