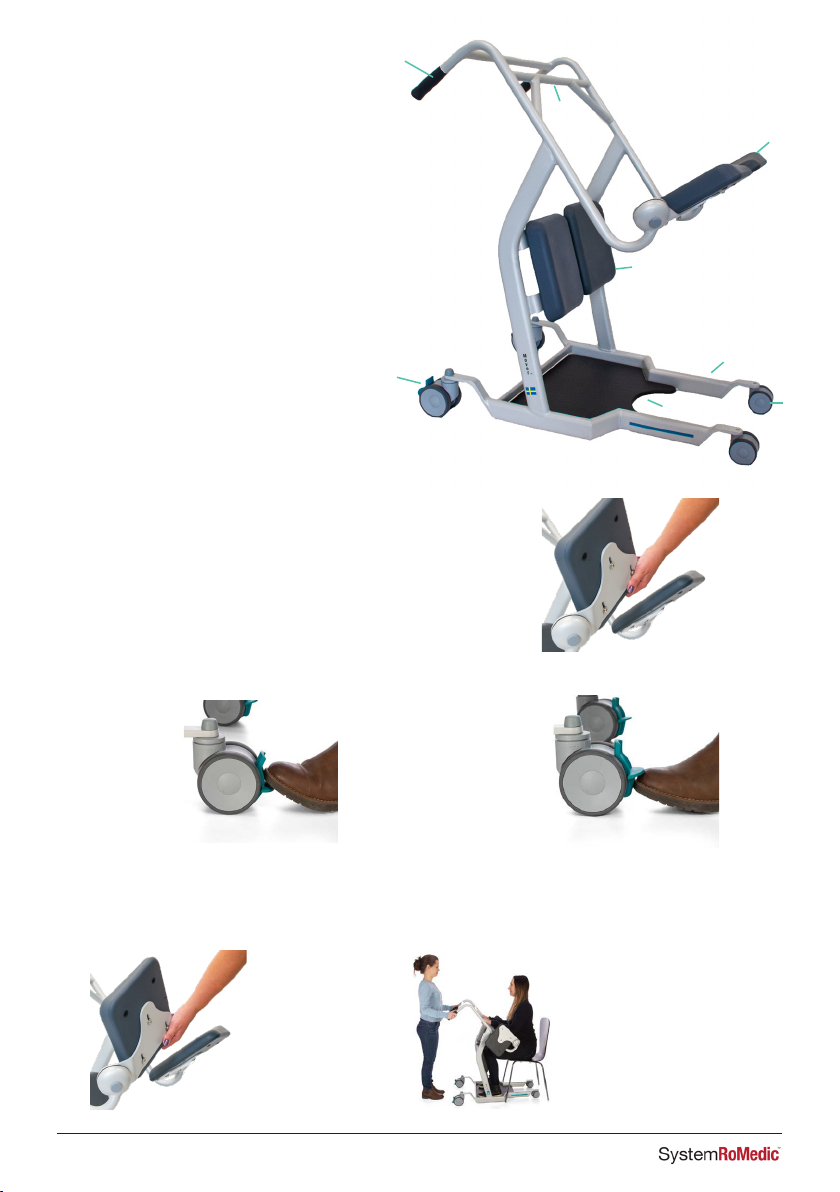
3. General Information
When passing over a threshold, pass the back wheel of the device first. Approach the threshold with lower
speed and communicate the coming threshold to the patient.
The product should only be used for the purpose specified in the IFU. Any other use is prohibited.
Keep the instructions for use together with the product so that it can be used as a reference if necessary.
Always carry out recommended daily maintenance before using the product.
Before using the product, a clinical assessment must be carried out as to whether the care recipient can be
moved using this product and, if so; whether the move/transfer should be carried out by one or two care givers.
Do not move a loaded device over obstacles that the castors cannot easily get over.
The product must be regularly cleaned/disinfected – after every use if necessary or at least once a day.
4IFU
Warnings
The caregiver must be able to read and understand the Manual/IFU of the product.
It is important to use only approved accessories to prevent unintended detachment of components and
subsequently a fall that may lead to patient injury.
Use careful and gentle maneuvers when moving the device.
Perform maintenance/service of the device, according to the instructions in the manual/IFU, at least once every
12 months.
Accessories must be properly fitted and tested in relation to the user’s needs and functional ability.
Special care must be taken when using strong electrical power sources such as diathermy and the like so that
diathermy cables are not placed on or near the device. In case of doubt consult with a Direct Healthcare Group
representative.
Do not leave a care recipient unattended during a transfer.
During a transfer or standup procedure, the user must be over the base of support of the device during the entire
transfer procedure, to avoid tipping of the lift.
Always maneuver the device using the handles. Do not push on the legs.
Repair any paint damage as soon as possible to avoid a shorter lifespan due to corrosion.
Do not use chlorine, phenols, pentanols or other strong detergents that could damage the surface/dry out the
cushions or cause premature cracking.
Do not allow the device to stand in direct sunlight since dark surfaces can become extremely hot.
The device must not be lowered into water.
The device must not be cleaned using steam.
The device must not be stored in a wet room.
The device must not be used outdoors, only indoors.
Please read these operating instructions carefully and contact us if you have any questions about the use or main-
tenance of the “Mover Aqua – Transfer Platform”.
4. Foreword
Please read the full instructions before using the product. It is important to understand the information in the
operating instructions so the product can be correctly used and maintained.
Keep the operating instructions with the product so they can be used as a reference whenever necessary. Ensure
that everyone who uses the product receives regular training in how to use the product. The product may not be
modified without the producer’s permission.