Therapeutix sm9128 User manual

Before operation, please read this user’s manual carefully, and be clear
about the instructions!
Contents:
Page
I.Foreword …………………………………………………………3
II.User manual …………………………………………………… 4
1.Warnings…………………………………………………………4
2. Precautions………………………………………………………4
3. Contraindication …………………………………………………6
4. Adverse Reactions …………………………………………………6
5. Indications for Use ………………………………………………7
6. Description of the Device …………………………………………7
6.1 General Description of the Device…………………………7
6.2 Specifications and Essential performance …………………8
6.3 Features ………………………………………………………9
6.4 Mode Description …………………………………………9
7. Direction for Use ………………………………………………13
7.1 General Operation Guidance………………………………13
7.2 Electrode Guidelines ……………………………………14
7.3 Regular TENS Application Principles……………………15
7.4 RegularTENSApplicationMethods ………………………16
7.5 Regular PMS Application Principles ……………………18
7.6 Regular PMS Application Methods ………………………18
8. Battery …………………………………………………………20
9. Readjustments, alterations and repairs …………………………21
10. Cleaning and maintenance …………………………………21
11. Storage…………………………………………………………22
12. Technical checks ………………………………………………22
13. Troubleshooting ………………………………………………24
14. Disposal of the Unit …………………………………………25
15. Warranty period ………………………………………………25
16. Electromagnetic Compatibility ………………………………25
17. Date……………………………………………………………30
III.Labels on the device ……………………………………………31
I. Foreword
Before operation, please read this user’s manual carefully, and be clear about the
instructions.
Twokey points foroperations:
1) Locate the exact location ofthe pain:Applythe pads (electrodes) tothe musclearea
where you are experiencing pain, stiffness or soreness.
2) Intensity: The intensity should be graduallyincreaseduntilyou reachthe highest
setting before it becomesuncomfortable.
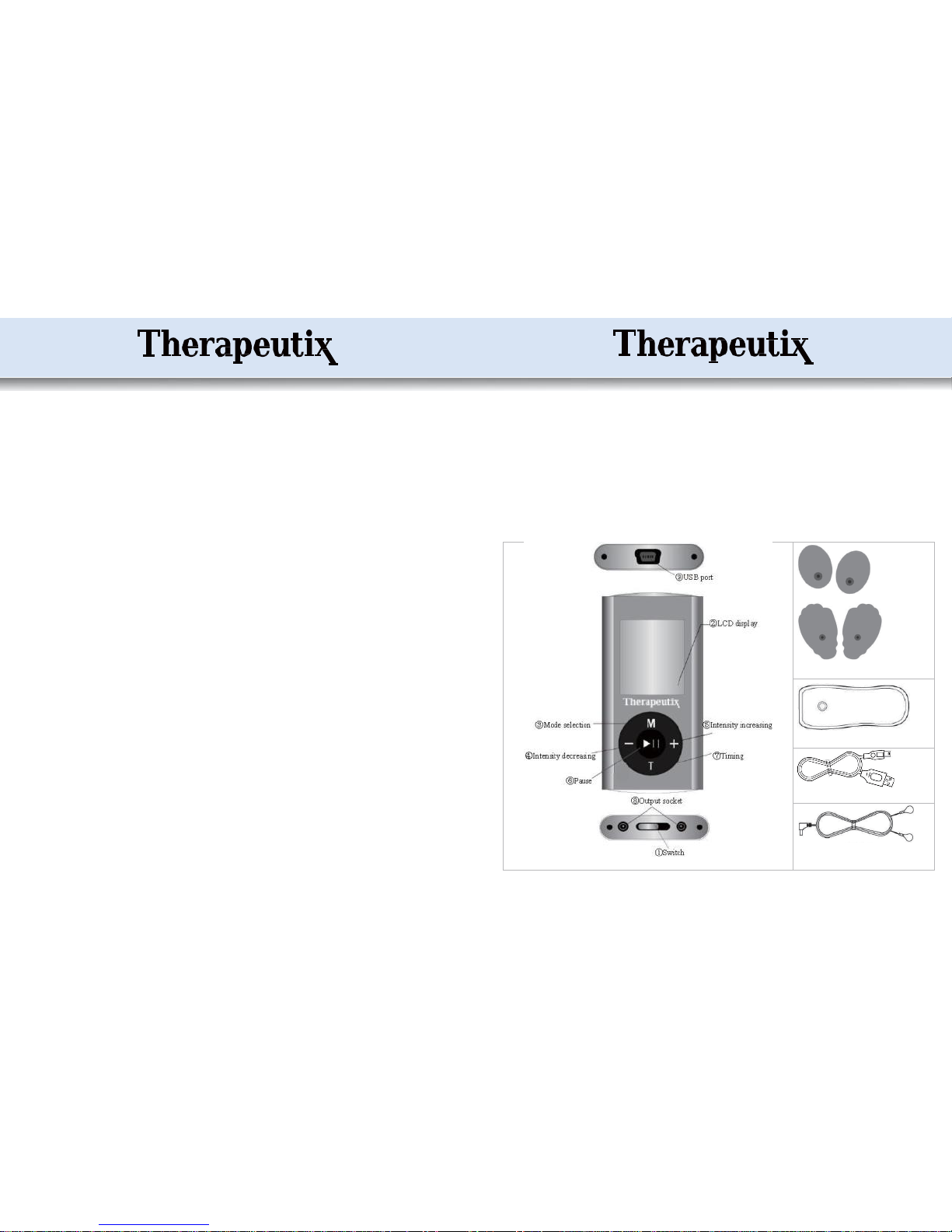
Structure andAccessories:
3
Accessories =>
Self-adhesive electrodes [510(k) cleared]
Battery charger
800mm (30.5 “) long USB cable
1500mm (59”) long Electrode cable
4
5
Included in thispackage:
*1 controller * 2 pairsof electrodes[510(k) cleared]
*2 output electrode cables * 1 USBcable
*1 charger * 1 User’smanual
*1 plasticholder
II. Usermanual
1. Warnings
1) The long-termeffectsofchronic electricalstimulation are unknown.
2) Do not use stimulation over the mainarteriesaround your neck.
3) Do not use stimulation over the neck or mouth. Severe spasmofmuscles may
occur and the contractions may be strong enoughto closethe airwayor cause
difficulty in breathing.
4) Do not apply stimulationdirectlyto the heartarea acrossthe chest or onthe chest.
5) Do not use stimulation onyour heador acrossyour head.
6) Do not use stimulation over swollen, infected,or inflamed areasor skin eruptions,
e.g., phlebitis, thrombophlebitis,varicoseveins.
7) Do not use stimulation over, or in proximityto, cancerouslesions.
8) Do not use stimulationwhencharging the device.
2. Precautions
1) Do not use the muscle stimulator during pregnancy.
2) Do not use if you have heart problems.
3) Do not use if you haveepilepsy.
4) Do not use the device in the presence ofthe following:
a.
When there is a tendencyto hemorrhage following acute trauma or fracture;
b.
Following recent surgical procedures when muscle contraction may disruptthe
healing process;
c.
Over the menstruating or pregnantuterus;
d.
Over areasof the skin which lack normalsensation.
5) Do not use the products near the heart, it may cause fast or irregular heartbeat.
6) Some patients may experience skin irritation or hypersensitivitydue to the
electricalstimulationor electricalconductive medium. The irritation can usually be
reduced by using an alternate conductive medium (like gel), or alternate electrode
placement.
7) Therapeutix TENS&PMS must be kept out ofthe reachofchildren.
8) Therapeutix TENS&PMS should be used onlywith the leads and electrodes
recommended for use by the manufacturer.
9) Never apply the padsto your skin with the power on, which will result in sudden
shock. If, during application, you want to move the padsto another body part, please
shut downthe device first,and then movethe padsto the place that you want to
stimulate.
10) Never use this product nearthe following devices: pacemakersor any other
embedded electronic medicaldevices, heart-lung machine and any other life keeping
electronic medical devices, electrocardiograph and any other medicalscreening and
monitoring devices. Simultaneoususe oftheTherapeutixTENS&PMS and any ofthe
abovedeviceswillcause malfunction and can be verydangerousto the users.
11) Two padsshould be used together as a pair. Always peeloff the protective film on
the pads before use. To avoid an electricalshort, do not connect two pads to each
other.
12) Do not applypadsto the same spot for over 60 minutes at atime.
13) Do not use theTherapeutixTENS&PMS while driving, operating machinery, or
duringany activityin which involuntarymuscle contractions may put the user at
undue risk ofinjury.
14) Do not usethe product in bathroomor a moist environment. Do not use while
bathing.
15) Do not use the product in the conditionofair mixed with flammablegases.
16) Never use this product in concurrence with high frequency surgical equipment;it
may result in burns at the site of the stimulator electrodes and possible damage to the
controlunit.

6
7
17) Never use this product near a microwave oven, or other highfrequency
equipment.
18) Never use this product while it is being charged.
19) Do not operate the product in close proximity (e.g. 1m/3ft))to shortwave
therapyequipment, it may produce instability in stimulator output.
3. Contraindication
1) Do not use this device on patients who have a cardiac pacemaker, implanted
defibrillator, or other implanted metallic or electronic device, because this may
cause electric shock, burns, electricalinterference, ordeath.
2) Do not usethisdeviceon patients whose painsyndromesare undiagnosed.
3) Do not use this device during pregnancy.
4) Do not usethisdeviceon babiesor infants who cannot expressthemselves.
4.Adverse Reactions
1) Patients may experience skin irritation and burns beneaththe stimulation
electrodes applied to theskin;
2) Patients may experience headache and other painful sensations duringor
following the application of electricalstimulation.
3) Patients should stopusing the device and should consult with their physiciansif
theyexperience adverse reactions from thedevice.
5. Indications forUse
TENS:
To be used for temporaryreliefof pain associated with sore and aching muscles in
the shoulder, waist, back, neck, upper extremities (arm), and lower extremities (leg)
due to strain from exercise or normalhousehold workactivities. Choose Mode 1, 3,
4, 5,6.
PMS:
It is intended to be usedto stimulate healthymuscles in orderto improve and
facilitate muscle performance. ChooseMode 1, 2, 3,6.
6. Description of theDevice
6.1 General Description of theDevice
Therapeutix TENS & PMS is a portable and DC 3.7Vbatterypowered multifunction
device, offering both TranscutaneousElectricalNerve Stimulation (TENS) and
Powered Muscle Stimulation (PMS) qualities in onedevice.
Therapeutix TENS & PMS has 6 operation modes, which can give certain electrical
pulses throughelectrode adhesive pads to the suggested areaof the bodywherethe
electrodes areplaced.
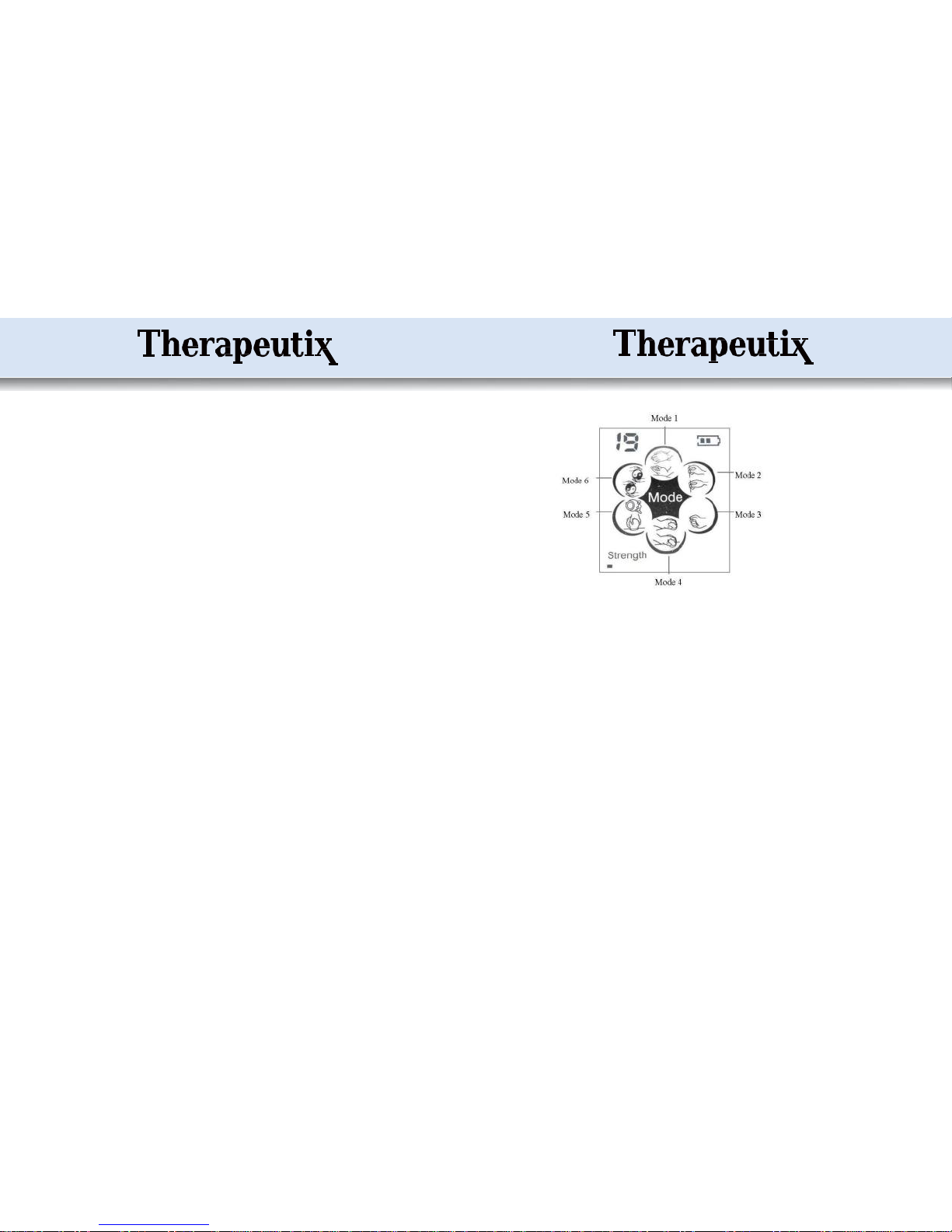
The electronic stimulatorymodule has the operating elements ofan ON/OFFSwitch,
Display screen, Mode Selection key, Intensity Modification keys, Timing key, Pause
key,Output socket,and USB port for batterycharging.
The displayscreencan show batterypower, selected mode, current intensity, time
remaining ofan application mode, and indication ofa pause(Page10, No. 10).
The device is equipped with accessories of electrode pads, electrode cables, a battery
charger, and one USB cable. The electrodecables are used to connect the pads to the
device; the USB cable is used to connect the charger and the built-in lithium battery.
All accessories, including USB cables, electrode pads, electrode cables, chargers can
only be changed or replaced by a qualified person.
The electrodesare interchangeable.The application areaof electrode pads must be
largerthanthe smaller electrodepads. The electrodepads are providedby GMDASZ
Manufacturing Co., Ltd. with 510(k) cleared Number K092546.
6.2 Specificationsand Essentialperformance:
(Essential performance: The values ofpulse duration, amplitudes, and repetition
frequencies do not deviate by morethan±30%whenmeasured with an error not
exceeding ±10% into a loadresistance (500Ω) within the range specified by the
manufacturer.)
8
9
-Power supply: DC3.7V
-Output voltage:42V@500Ω
-Output current:84mA@500Ω
-Consumed current: 40mA
-Pulse width: 100μS
-Frequency: 1~110Hz
-Smallest are of electrode pad:4cm2
-Timer: 10~60 minutes
-Strength level adjustment: 20grades
-Charger: Input: 100-240V 50/60Hz 0.18A Max; Output: DC5V 250mA;Class
II, not appliedpart,not suitable for use in the presenceofa flammablegases,
with oxygen or nitrousoxide, or for continuousoperation..
6.3 Features
*Alarge, easyto read LCD display
*Adjustable timer
*6 Modes
*Rechargeable lithiumbattery
6.4 ModeDescription
We suggestedthat you initially experiment using each ofthe 6 modes.The modethat
gives you the most desirablesensations and comfort is the most appropriateone to use
for your currentcondition.
1) Intensity should be set at a levelwhere you will feelsome muscular vibration and
involuntary muscle movements.
It should be set to give acomfortable feelingand should not be painful.
2) Timingofapplicationshould be at least 10 to 20 minutes.
3) Frequencyofthe application should be at least one to threetimesper day.
10
11
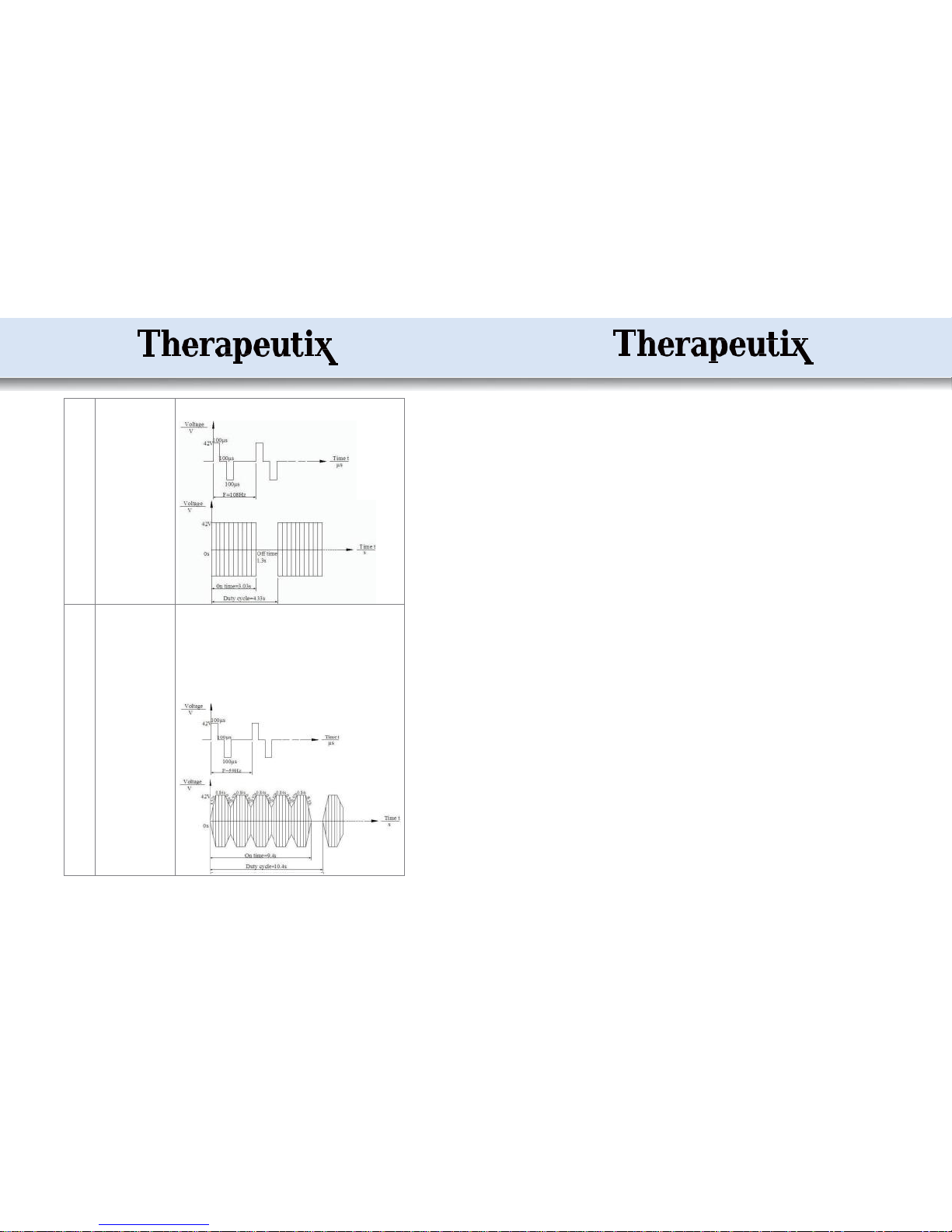
Mode 3
1)
Timing: 10~60 minutes
2)
Pulse train frequency:
1.17Hz
3)
Pulse width: 100μS
4)
On time: 0.7ms
5)
Off time: 849.3ms
6)
Duty cycle: 850ms
The device outputs 2 groups of symmetrical pulse at intervals of 0.85 seconds. The
pulse width is 100μS fixed.
Mode 4
1)
Timing: 10~60 minutes
2)
Pulse train frequency:
5.813Hz
3)
Pulse width: 100μS
4)
On time: 3.02s
5)
Off time: 1.1s
6)
Duty cycle: 4.12s
The device outputs 2 groups of symmetrical pulse at intervals of 0.172s.
The pulse width is 100μS fixed. The whole waveform works for 3.02s and stops for
1.1s. The device repeats this cycle all the time.
Modes
Parameters
Graphical Description
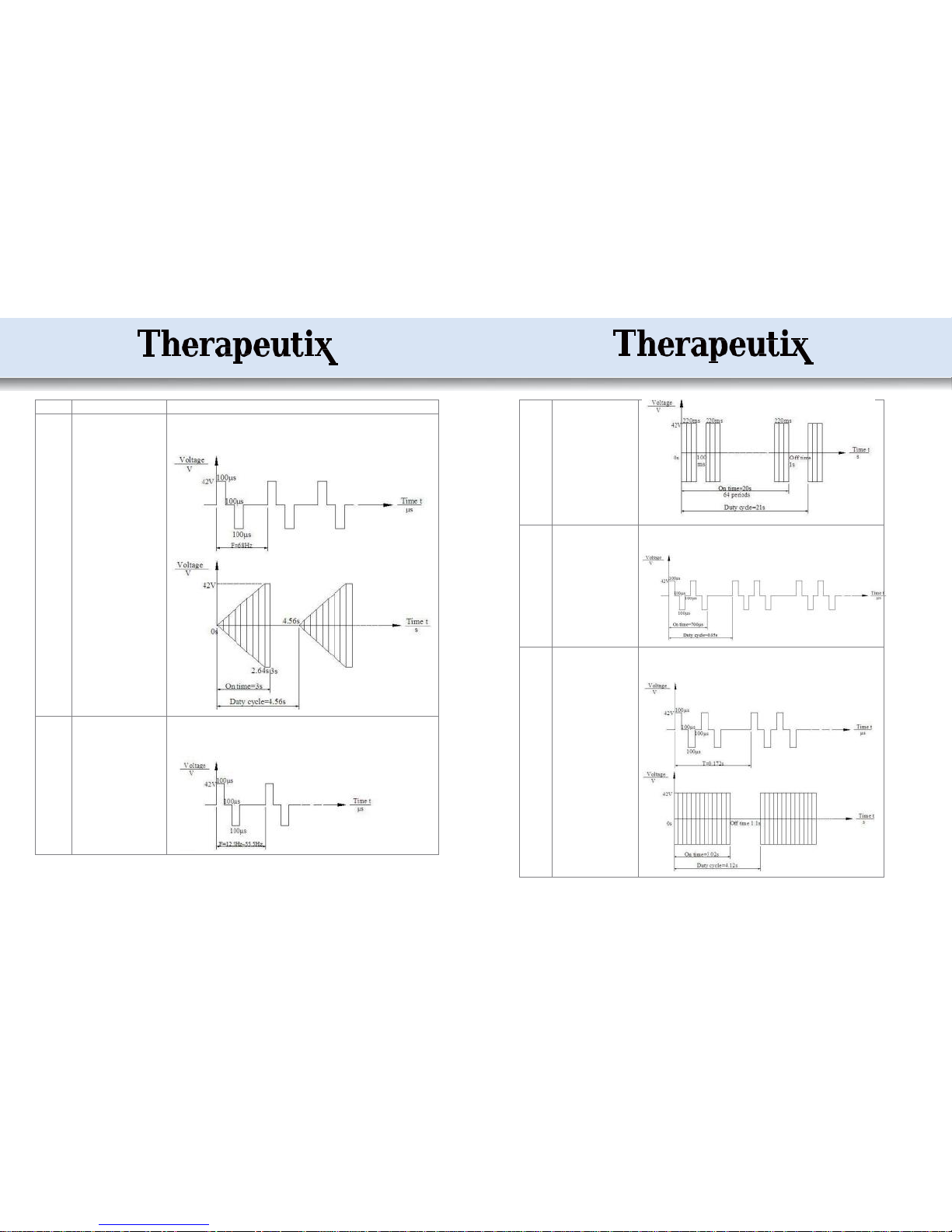
Mode 1
1) Timing: 10~60 minutes
2) Frequency: 68Hz
3) Pulse width: 100μS
4) On time: 3s
5) Off time: 1.56s
6) Duty cycle: 4.56s
The amplitude for the whole pulse takes 2.64 seconds from zero to maximum and
stays on for 0.36 seconds; then the device stops for 1.56 seconds. The device repeats
this cycle all the time. The output frequency is 68Hz and the pulse width is 100μS.
Mode 2
1) Timing: 10~60 minutes
2) Frequency: 12.5~55.5Hz
3) Pulse width: 100μS
4) On time: 20.S
5) Off time: 1s
6) Duty cycle: 21s
The output amplitude and pulse width (100μS) are fixed; the frequency changes
among 12.5Hz to 55.5Hz; and there are 64 periods (on 220ms, off 100ms), then the
device stops for 1 second. The device repeats this cycle all the time.
12
13
7. Direction forUse
7.1 General OperationGuidance
1) The Therapeutix TENS & PMS unit needs to be chargedfor up to 10 hoursbefore
the firstuse.
2) Connect the electrodecablesto the output sockets at the bottomofthe unit.
3) Connect the pads to the cables by snapping them on.
4) Use a damptowelto wipe the skin where you intend to put the pads, so as to
remove any bodyoil, cosmetic or dirt. Removethe protective filmand place the pads
on the area making sure that both pads are on the skin and notoverlapping.
Warning:To avoid an electricalshort, never put two electrodepadstogether.
5) Turnonthe unit by sliding the On/Offswitch fromOffto On.
Warning:Do not move pads ortouchthemwith your hands during use, it may cause
strong stimulation.
6) Whenyou haveturnedon the unit, the LCD display will automaticallyshow Mode
1. The “T” (Timer) willautomaticallychoosethe time durationof20 minutes.
7) To change the modes, press the M (Mode) button. Once you have selected a mode,
gradually increase the intensity by pressing the + button, and to decrease the intensity
by pressing the –button. The chosen power output depends on your comfort level. At
the precondition of acceptance, the intensity should be chosen as high as possible for
the best effect but still feelcomfortable.
8) Toset the timeofuse, pressthe T button. The auto time set is 20 minutes. Each
pressofthe T buttonincreases the time by 10 minutes.After the time runs out the
deviceturnsoffautomatically, and it can be restartedif treatment is needed to be
continued.
9) Sit backand enjoythe deep soothing sensations!
Notes:The Therapeutix TENS & PMS unit is very safe, the output intensity increases
only bypushing the + key. Even if the intensityis increasedto the maximum, it is
withinthe safe range,but may feeluncomfortable. Whenthe userswitchesthe mode,
the intensitywillautomaticallygo downto the minimumfor safetyreasons.
10) Pressthe Pausekey(“►II” button)to lockthe LCD display, the “MODE” display
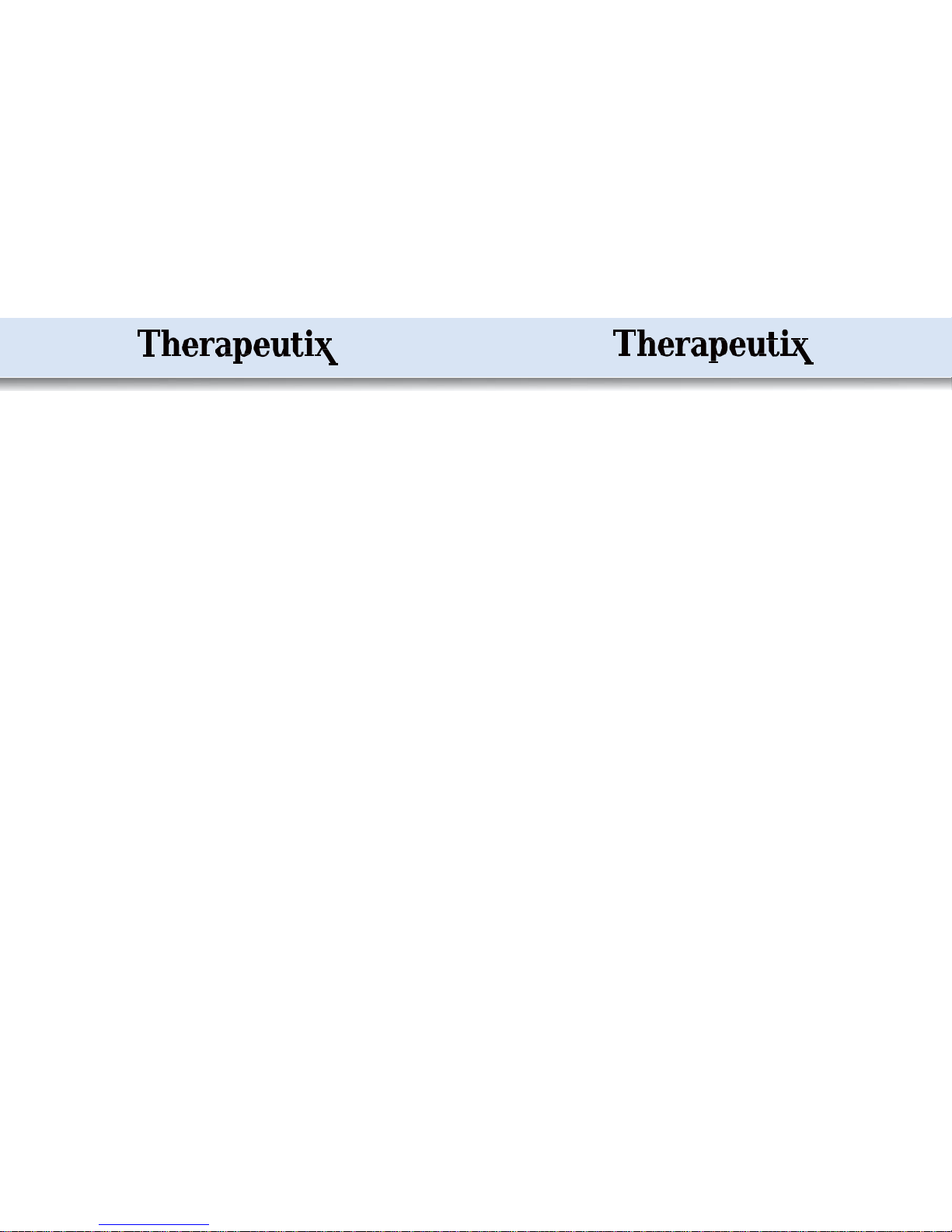
Mode 5
1) Timing: 10~60 minutes
2) Frequency: 108.2 Hz
3) Pulse width: 100μS
4) On time: 3.03s
5) Off time: 1.3s
6) Duty cycle: 4.33s
The output frequency (108Hz), amplitude and pulse width (100μS) are fixed, on for
3.03 seconds and off for 1.3seconds. The device repeats this cycle all the time.
Mode 6
1) Timing: 10~60 minutes
2) Frequency: 59Hz
3) Pulse width: 100μS
4) Build-up phase: 0.52s
5) Working time: 0.84s
6) Run-down phase: 0.52s
7) Pause phase: 1s
8) Duty cycle: 10.4s
The output frequency (59Hz) and pulse width (100μS) do not change; the amplitude
for the whole pulse takes 0.52s from zero to maximum and stays for 0.84s; then it
takes 0.52s from maximum to a half.
Then the device repeats 3 times of the following: the amplitude for the whole pulse
takes 0.52s from a half to maximum and lasts for 0.84s; then it takes 0.52s from
maximum to ahalf.
Then the amplitude for the whole pulse takes 0.52s from a half to maximum and
lasts for 0.84s; then it takes 0.52s from maximum to 0, and stops for 1s.
The device repeats the above cycle all the time.
14
15
willblink.Thedevicewill not workno matter what key you pressonthe control
panel,it is locked.To unlockpress the Pause key again.
11) Ifyou need to turnoffthe device duringstimulation, slide the On/Offswitchto
“OFF”.
12) Before storing the TENS &PMS use the protective film to coverthe electrode
pads.
Notes:
①Never connectsthisproduct with a common headphone.
②Please do not touchthe USB port whenusing thedevice.The USB port is only
used to connect the charger, do not connect other devices.
③The charger supplied bythe manufacturer mustin compliancewith IEC/EN
60601-1, the use ofunauthorizedchargerscan impair the safety.
④The batteryneeds to be chargedfor upto 10 hoursbeforethe first use.
7.2 Electrode guidelines
1) Use only the electrodessupplied bythe manufacturer;other electrodesmay
present a risk of unsuitable electricalcharacteristics with yourstimulator.
2) Do not use the electrodesondifferent people, otherwise, skin reaction or cross
contamination mayoccur.
3)Always turn power off before removing or repositioning theelectrodes.
4) Washskinthoroughly, and thendry it before applying the electrodes.
5) Applythe whole surfaceofthe electrodesfirmlyto the skin. Do not use electrodes
that do not stick properlytothe skinor only partially stick to the skin.
6) In case ofskin redness under the electrodesafter a stimulation session, do not start
a new session in the same place if skin redness is stillevident.
7.3 Regular TENS Applicationprinciples
1) Find the exact pain point or the area where the muscles ache most. For best
relief of pain, place the electrode pair from one channel on either side of the pain.
(See Figure 1: Twin mode) or you may place one electrode on the painful siteand
the other near the site ofpain.
2) Intensity: The intensity can be gradually increased up to the point when it
becomes uncomfortable. Always stay below that point ofdiscomfort.
3) Recommended application duration and Mode selection:
When starting out, choose Mode 1 at a low Intensity level for 10 minutes up
to 3 times a day. You may increase the intensity and time after youhave
become familiar with the device and the feel for the stimulation. Staywith
Mode 1 for a few days before trying any of the other Modes and intensity
settings. Remember, the modes to be used for pain relief are Modes 1, 2,3, 4,5
and 6.
It is difficult to recommend a particular mode for a specific type of pain andit
is usually determined by the user’s feel of relief.
However, if you do not feel any relief of pain after having tried different
modes and intensities it is recommended that you consult with your physician.
4) If the stimulation sensation becomes weaker or disappears, you may increase
the intensity by pressing the up key (+) to a point when the stimulationbecomes
uncomfortable, but if the sensation does become uncomfortable, press thedown
key (-) to decrease the intensity. Always stay under the point of discomfort!
5) If you experience an adverse reaction (skin irritation/redness/burns /other
painful sensation), or if you feel unusual discomfort, stop using thedevice
immediately.
6) There are two ways to place the pads, in twin or opposed modes.
Fix the two pads from one channel on either side of the pain area, or one
electrode on the painful site and the other near the site of pain, but on thesame
side of body. If you want to use both channels at the same time, make sure that
the second pair of electrodes is also fixed near the side of pain on the same side
of the body (right under or over the first pair)
16
17
Figure 1: Twin mode (this is the correct mode)
Fixing the two or four pads respectively on the opposite sides ofthe
body is not recommended and provides little benefit.
Figure 2: Opposed mode (Not recommended)
7.4 Regular TENS ApplicationMethods
Use the device for temporary relief of pain associated with sore and aching muscles
in the shoulder, waist, back, neck, upper extremities (arm), and lowerextremities
(leg) due to strain from exercise or normal household work activities.
Users can choose Mode 1, 2, 3, 4, 5 or 6 for temporary relief ofpain.
*Many people experience immediate relief from muscle pain, while othersrequire
several days of regular use to feel the benefits. The results vary and willdepend
upon your underlying conditions and how often you use the device.
If your pain does not improve, you can try to increase the intensity and time or
change the mode.
Note:
The charts below are merely a suggestion for whereto placethe electrodes, what
Modeto chooseand how longto stimulate, but only afterthe userhas gone
through the starting procedure (above)and is familiarwith the device.
●
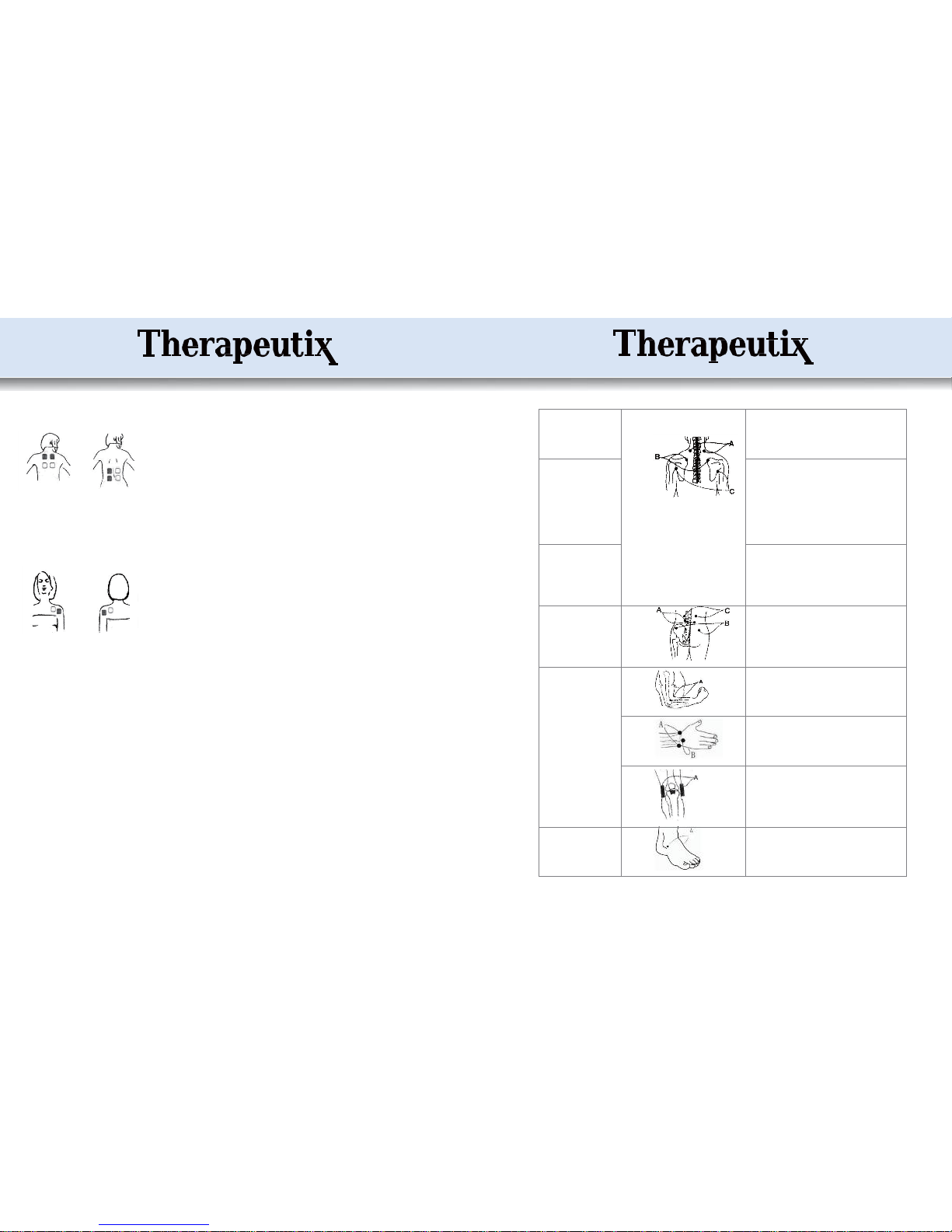
Pain in Neck
Figure 3:
Mode 1 (A) for 10-20 minutes, and Mode 3 for 10-20
minutes; twice or 3 times a day.
* Keep the neck warm and avoid sleeping on a high
pillow.
●
Pain in shoulders
Mode 4 (B & C) for 10-20 minutes, and Mode 5 for
10-20 minutes; twice or 3 times a day.
*
Find the pain area and apply the electrodes at the
anterior and posterior (inner & outer) shoulders.
*
Keep the area warm. Avoid sudden movements with
the aching shoulders, gentle movements are advisable
in the initial stage and full motions at a later stage.
●
Pain in Back
Mode 1 for 10-20 minutes, and Mode 5 or 6 for 10-20
minutes; twice or 3 times a day.
*
Apply the electrodes to the pain area.
*
Avoid working in the same position in the initial
phase and change the position at times.
●
Pain in Waist
Figure 4:
Mode 3 or 6 to stimulate (A) for 10-20 minutes,
Mode 1 to stimulate (B and C) for 10-20 minutes;
twice or 3 times a day.
●
Pain in Joints and limbs
Figure 5:
Mode 1 for 10-20 minutes, and Mode 4 for 10-20
minutes; twice or 3 times a day.
Figure 6:
Mode 3 for 10-20 minutes, and Mode 4 for 10-20
minutes; twice or 3 times a day.
Figure 7:
Mode 1 for 20 minutes and Mode 3 for 20 minutes;
twice or 3 times a day.
Figure 8:
Mode 4 for 10-20 minutes, and Mode 6 for 10-20
minutes; twice or 3 times a day.
* Do not use this device directly on the pain area or
area of injury.

18
19
7.5 Regular PMS Applicationprinciples
1) Identify the targeted muscle which needs to be stimulated. In order toimprove
or facilitate muscle performance, place the two electrodes from one channel on
opposite ends of the muscle or close to the belly of the muscle. (See Figure9)
If you like to work with both channels at the same time you may place the second
electrode pair near the first pair or onto another muscle to be stimulated likethe
biceps on the opposite arm in Fig 9below.
2) Intensity: The intensity can be gradually increased up to the point when it
becomes uncomfortable. Always stay below that point ofdiscomfort.
3) Recommended application duration and Mode selection:
The modes to be used for improving the muscle performance are Modes 1, 2, 3
and 6.
Mode 1: exercise preparation
Mode 2: Build endurance
Mode 6: muscle strengthening
Mode 3: Activerecovery
7.6 Regular PMS ApplicationMethods
This function is intended to stimulate healthy muscles includingabdomen
muscles in order to improve or facilitate muscle performance.
Youcan use the electrode adhesive pads on almost every muscle and joint area on
your body. Please note that this device is intended to stimulate healthy muscles
in order to improve or facilitate muscle performance. It is not intended as
therapy for any medical condition.
Users can choose Mode 1, 2, 3 or 6 to stimulate the following points toquickly
facilitate muscle performance. In order to better improve the muscle
performance, you may increase the intensity gradually to a level which isstill
comfortable and does not cause pain or discomfort. Furthermore, you should
use the Therapeutix TENS & PMS regularly to maintain the benefit you may
have gained during exercise.
Note:
The charts below are merely a suggestion for how to place the electrodes,
whatModeto chooseand how long to stimulate,only afterthe userhas gone
through the starting procedure(above)and is familiarwith the device.
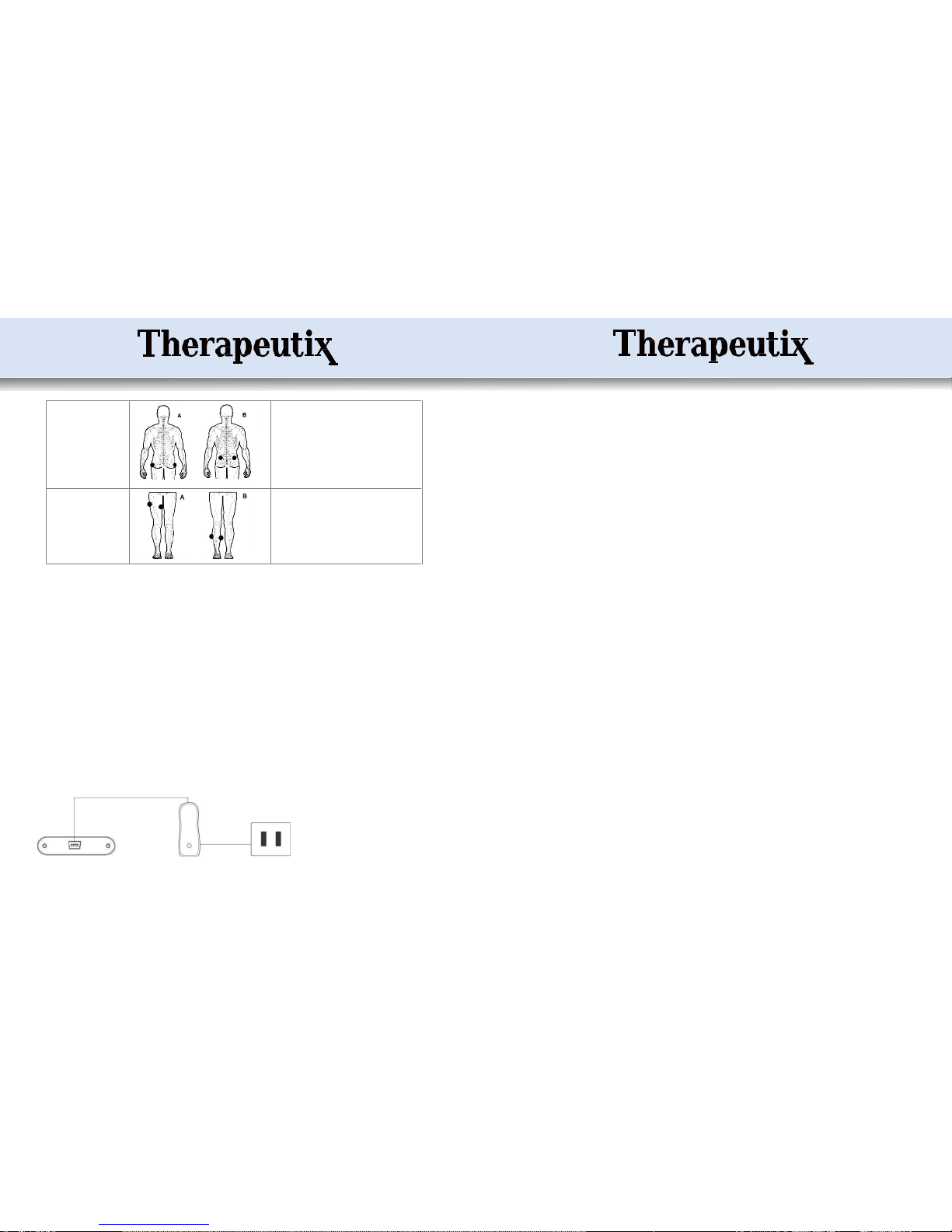
Targeted
muscle
Figure9:
●
Abdomen
Mode 1 for 10-20 minutes, Mode 2 for 10-20
minutes, and Mode 6 for 10-20 minutes; twice or
3 times aday.
* Persistent and consistent use can help to
improvetheabdomenmuscles.
●
Waist
Mode 1 for 10-20 minutes, Mode 3 for 10-20
minutes, and Mode 6 for 10-20 minutes; twice or
3 times aday.
Thedeviceexercisesthemusclesinthe waist
* It is advisable to do some waist exercise after
the application.
●
Shoulders and Back
Mode 1 for 10-20 minutes, and Mode 2 or 3 for
10-20 minutes; twice or 3timesa day.
Apply the electrodes to the points shown to
exercisetheseshouldermuscles
20
21
●
Buttocks
Mode 1 for 10-20 minutes, and Mode 6 for
10-
20 minutes;twice or 3 times aday.
Apply the device to the points shown to exercise
the muscles.
●
Legs
Mode 1 for 10-20 minutes, Mode 2 for 10-20
minutes, and Mode 3 for 10-20 minutes; twice or
3 times aday.
The device can stimulate areas of the legs and
thighssoas toimprovethemuscles.
8. Battery
8.1 Battery information
Capacity: 110mAh
Voltage: DC 3.7V
Restriction: 4.2V
8.2 Charging the Battery
1) The Lithium battery can be recharged through both AC adaptor andcomputer
USB input.
2) Turn off the unit.
3) Connect the unit and the charger with USB extension cord. Plug chargerinto
any power outlet, a green light shows that it is charging. The chargingprocess
will last approximately 1 hour. When charging is finished, the LCD will show a
full battery cell.
4) The battery should be charged for 10 hours or so before first use. We have
done this for you!
Notes: Only charge the unit when battery is completely drained the first 2
times. Unplug the charger from power outlet when charging is complete.When
stimulation intensity decreases, it indicates that the device needscharging.
Recharge it and then continue to use the device. Do not use the devicewhile
charging.
9. Readjustments, alterations and repairs
1) Do not disassemble, repair or modify the device without authorization, you
will void any warranty on the product.
2) The manufacturer is only responsible for the safety and performance of
Therapeutix TENS & PMS when readjustments, alterations and repairs are
carried out by authorized individuals and when the Therapeutix TENS &PMS
is used in accordance with the user instructions.
3) Qualified technicians who are familiar with the technical features ofthe
device have been provided with circuit diagrams, PCB drawings, component
lists and setting instructions by the manufacturer.
10. Cleaning and maintenance
A.
For the control unit:
1) To keep the controller clean, use a soft and dry cloth for dust or a soft damp
cloth for any dirt and smudges. Do not use any cleaning solutions to clean the
controller and its pads.
2) Do not use or store the device where there are magnetic fields or electric
waves (near TV set or speakers).
3) Do not place the devices in areas of high temperature, high humidity,or
under direct sunlight.
4) Keep the device out of reach of children.
5) All worn accessories should be disposed of according to your local
regulations.
B.
For electrode pads:
22
23
* Refer to the user manual of the pads manufacturer (510K092546), or
reference to the following suggestions:
1) Unplug the output cord from the output jack of the controller after each
use. Cover both pads with the protective film before storage. Never foldthe
electrode pads.
2) Between uses, store the electrodes in the reusable bag in a shady place.
Stockroom temperature: +5°C~+27°C (41-80°F) and humidity of30%~80%.
No need to sterilize.
3) Never apply the pads to any other surface other than your skin. If thepads
become soiled or dirty, the adhesive power may decrease. In thiscase,
moisten the surface of the pads with water and wipe away the dirtyportion.
This will allow a temporary restoration of the adhesive power. However, too
much water will result in loss of the adhesive power.
4) The life of the electrodes varies depending on skin conditions, storage,
amount of use, type of stimulation, and stimulation site. Electrode life may be
extended by carefully following this Instruction for Use. The expired
electrodes are to be recycled and do not harm environment.
Warning: The electrodes are intended for single patient useonly!
11. Storage
Caution: Do not store in a damp area. Dampness may affect the deviceand
cause rust.
-Normal working ambient temperature: 5°C~40°C (40-104°F)
-Normal working ambient humidity: ≤80%RH
-Store and transport ambient temperature: -20°C~55°C (4° -131° F)
-Store and transport ambient humidity: ≤93%RH
12. Technical checks
Technical checks on the device should be performed every 24months.
These include:
1. Checking to see whether the user instructions and the medical device book are
included in the accompanying documentation.
2. Checking the equipment for completeness.
3. Visual check:
-for mechanical damage
-for damage to all cables and connections
4. Functional safety
-Checking the output signals with a load resistance of 500Ω real (current and
voltage)
-Checking the frequency
-Checking the pulse width.
These technical checks may only be performed by individuals with appropriate
training. The results must be noted in the medical device book along with thedate
and name of the person carrying out the check.
24
25
13. Troubleshooting 14. Disposal of the Unit
To dispose of the unit, its accessories and packing materials, take appropriate
actions in accordance with the rules and regulations in force in your area to
prevent adverse ecological effects.
15. Warranty period
Weoffer a lifetime warranty from the date of purchase on Therapeutix
TENS Unit Electronic Massagers. This warranty does not cover cables and
electrodes.
16. Electromagnetic Compatibility
Important information regarding Electro Magnetic Compatibility (EMC)
With the increased number of electronic devices such as PC andmobile
(cellular) telephones, radio transceivers, mobile radio transmitters,
radio-controlled toys, and so on, Medical devices in use may be susceptibleto
electromagnetic interference from other device. Electromagnetic interference
may result in incorrect operation of the medical devices and create a
potentially unsafe situation. Medical devices should also not interferewith
other devices.
In order to regulate the requirements for EMC (Electro Magnetic
Compatibility) with the aim to prevent unsafe product situations, the
EN60601-1-2 standard has been implemented. This standard defines thelevels
of immunity to electromagnetic interference as well as maximum levels of
electromagnetic emissions for medical devices.
This unit has been thoroughly tested and inspected to assure proper
performance and operation! This product needsspecial
precautions regarding EMC and needs to be installed and put into service
according to the EMC information provided,
the following tables recommend minimum separation distances between
portable and mobile RF communications equipment and the TENSunit.
Problems
Possible causes
Try thissolution
No.
Onepad feelsstrongerthanthe
other.
Thisisnormal. Different areas of your
bodywillreactdifferently.
Nothingneeds to be done. Make surethe
pads are sticky and are making good
contact.
1
The intensity is notfelt.
Veryweakintensitylevel.
Pads arenot attached tothe bodyfirmly.
Attach both pads firmly totheskin.
Thetransparent films are stillstuck to
thepads.
Peel off film onthe adhesive surface of
pads.
Thepadsstackedtogetheroroverlap.
Donot stack pads together oroverlap
pads.
The cord is not properly connected tothe
unit.
Connectcordcorrectlyintothejack.
Theintensitysettingisgettingweak.
Increasetheintensitylevel.
Thebatterycapacityislow.
Chargethebattery.
2
The skin turns red orthe skin
feels irritated.
The adhesive surface of padsisdirtyor
dry.
Wash adhesive surface of pads softly
with your fingertips for about 3seconds
underslowrunningwater.
The therapy time is too long or the
intensityissettoohigh.
Reducethe applicationtimeorreduce
the intensity.
Theelectrodepad surfaceisworn out.
Replaceelectrodepad.
3
Nopower source; nodisplayin
LCD.
Thebatterycapacityisdepleted.
Chargethebattery.
4
Power cut off during use.
Thebatteryisweak.
Chargethebattery.
Thecordisbroken.
Replacethecord.
5
Itis difficult toattach the pad
to theskin.
Haveyouremoved the transparentfilm
from thepad?
Peel off film onthe adhesive surface of
pads.
Wasthe pad applied immediately after
washing?
Dry thepad.
Isthe adhesive surface ofthe pad
damaged?
Replacethepad.
6
Adhesive surface ofpad is not
sticky.
Areyouusingpadwhenperspiring?
Use when not perspiring,in a coolroom.
Were the pads stored under high
temperature,highhumidity,ordirect
sunshine?
Replacethepad.

26
27
Caution:
*The use of accessories and cables other than those specified byTherapeutix,
with the exception of cables sold by Therapeutix as replacement partsfor
internal components, may result in increased emission or decreased immunity
of the device.
*Do not use a mobile phone or other devices that emit electromagnetic fields,
near the unit. This may result in incorrect operation of the unit.
*This device should not be used adjacent to or stacked with otherequipment
and that if adjacent or stacked use is necessary, this device should be observed
to verify normal operation in the configuration in which it will be used.
*Refer to further guidance below regarding the EMC environment inwhich
the device should be used.
There is no guarantee that interference will not occur in a particular
installation. Radiated or conducted electromagnetic signals can cause:
1) As to devices:
•Deviation of the values of pulse duration, amplitudes, and repetition
frequencies, may impair the unit’s essential performance. The device has
passed EMC highest interference level test, and the parameters do not deviate
the essential performance requirement.
•The device displays abnormally in LCD.
2) As to patients:
•The sensitivity of stimulation may be weaker or stronger, but it does not
produce safety issues.
•It cannot achieve expected effect.
If this equipment is found to cause or respond to interference, attempt to
correct the problem by one or more of the following measures:
•If feeling too weak or too strong stimulation, adjust the strength level to an
acceptable level.
•If the device display is abnormal, power off and restart the device and check
whether it shows properly.
•Re-orient or re-locate the affected device.
•Increase the separation between the unit and the affected device.
•Power the equipment from a source other than that of the affected device.
•Consult the service representative for further suggestions.
1) Guidance and manufacture’s declaration –electromagneticemission
The Therapeutix TENS & PMS is intended for use in theelectromagnetic
environment specified below. The customer of the user of the Therapeutix
TENS & PMS should assure that it is used in such anenvironment.
Emission test
Compliance
Electromagnetic environment –guidance
RF emissions CISPR 11
Group 1
The Therapeutix TENS & PMS uses RF energy only
for its internal function. Therefore, its RF emissions
are very low and are not likely to causeany
interference in nearby electronic equipment.
RF emission CISPR 11
Class B
The Therapeutix TENS & PMS is suitable for usein
all establishments, including domestic establishments
and those directly connected to the public low-voltage
power supply network that supplies buildings usedfor
domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations / flicker
emissions IEC61000-3-3
Complies

28
29
2) Guidance and manufacture’s declaration –electromagnetic immunity 3) Guidance and manufacture’s declaration – electromagnetic immunity
TheTherapeutixTENS&PMSisintendedforuseintheelectromagneticenvironmentspecifiedbelow.Thecustomerortheuserof
TherapeutixTENS&PMSshouldassurethatitisusedinsuchanenvironment.
Immunity test
IEC 60601test level
Compliance level
Electromagnetic environment - guidance
Conducted RF
IEC61000-4-6
3 Vrms
150 kHz to 80MHz
3Vrms
Portable and mobile RFcommunications equipment should
be used no closer to any part of the Therapeutix
TENS & PMS, including cables, than the recommended
separation distance calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
d 1.167 P
d 1.167 P 80 MHz to 800 MHz
d 2.333 P 800 MHz to 2.5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d istherecommended separation distance
in meters(m).
Field strengths from fixed RF transmitters, as determined
by an electromagnetic site survey,
a
should be less than the
compliancelevelineach frequencyrange.
b
Interference mayoccur inthevicinity ofequipment marked
withthefollowing symbol:
Radiated RF
IEC61000-4-3
3V/m
80MHz-2.5 GHz
3V/m
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflectionfrom structures,objects andpeople.
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the
electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the
measured field strength in the location in which the Therapeutix TENS & PMS is used exceeds the applicable RFcompliance
level above, the Therapeutix TENS & PMS should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as reorienting or relocating the Therapeutix TENS & PMS. Over the
frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
The Therapeutix TENS & PMS is intended for use in the electromagnetic environment
specified below. The customer or the user of Therapeutix TENS & PMS should assure
that it is used in such an environment.
Immunitytest
IEC 60601 testlevel
Compliance level
Electromagnetic environment - guidance
Electrostatic discharge
(ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floorsshouldbe wood,concreteorceramictile.
If floor are covered with synthetic material,
therelativehumidityshouldbeatleast30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±2kV for power
supply lines
Mainspowerqualityshould be thatof atypical
commercial or hospital environment.
Surge
IEC 61000-4-5
±1kVline(s)toline(s)
±1 kV differentialmode
Mains power quality should be that of a typical
commercial or hospital environment.
Voltage dips, short
<5% UT
<5% UT
interruptions and
(>95% dip in UT)
(>95% dip in UT)
voltage variations on
power supply input lines
IEC 61000-4-11
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
Mains power quality should be that of a typical
commercial or hospital environment. If the user
of the Therapeutix TENS & PMS requires
continued operation during power mains
interruptions, it is recommended that the
70% UT
(30% dip in UT)
70% UT
(30% dip in UT)
Therapeutix TENS & PMS be powered from an
uninterruptible power supply or a battery.
for 25 cycles
for 25 cycles
<5% UT
<5% UT
(>95% dip in UT)
(>95% dip in UT)
for 5 sec
for 5 sec
Power frequency
(50Hz/60Hz) magnetic
field IEC 61000-4-8
3A/m
3A/m
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typical commercial or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
30
31
4) Recommended separation distances between portable and mobile RF
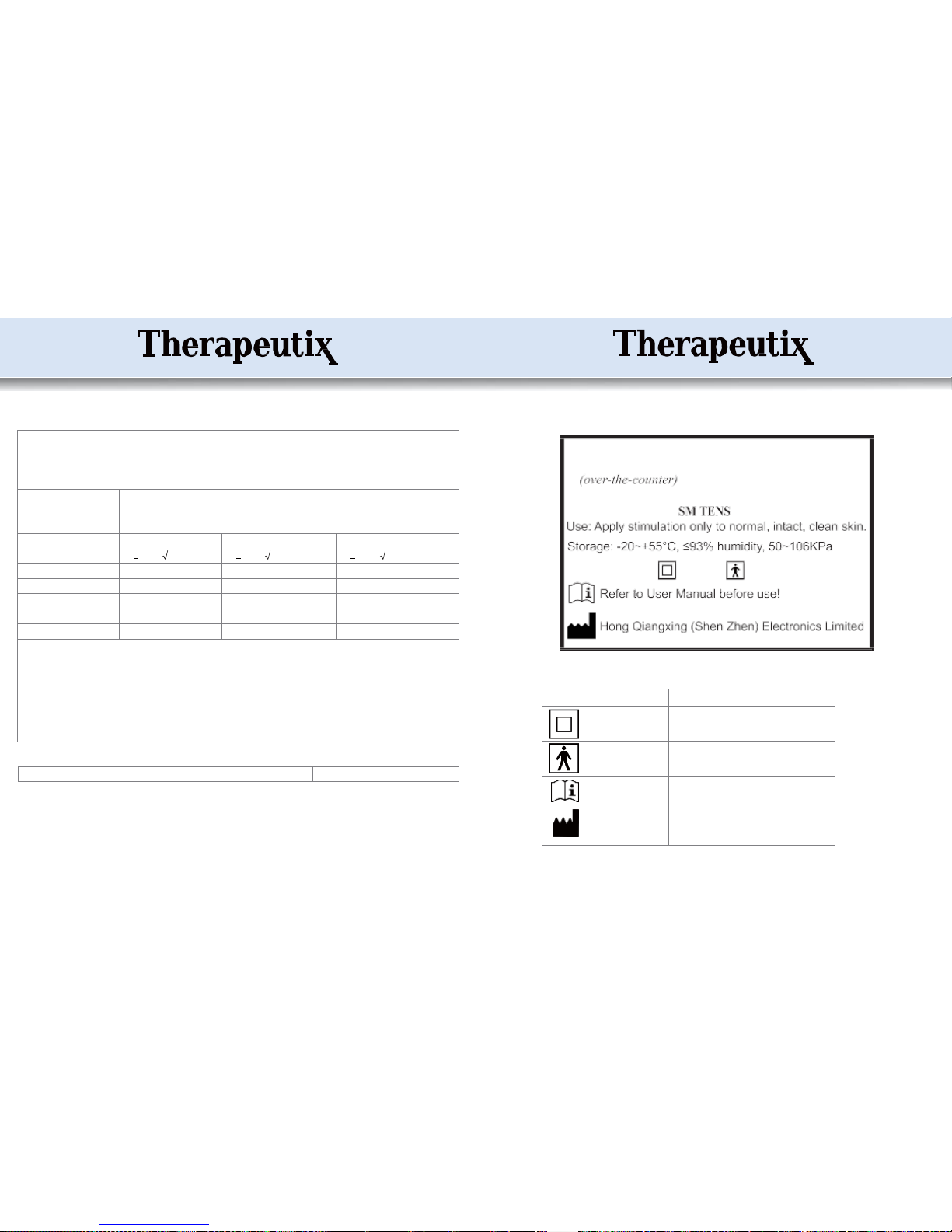
communications equipment and the Therapeutix TENS & PMS III. Labels on the device
Notes:
17. Date
Issue date of the manual: 12-25-2014
Production date: 12-30-2014
Batch: xxx-xxx
Therapeutix
Model:SM9128
lnput:DC 3.7V
Weight:38.6g
Size:86x43.2x10.6MM
TheTherapeutixTENS& PMSisintendedforuseinan electromagneticenvironmentinwhichradiatedRF disturbancesarecontrolled.
ThecustomerortheuseroftheTherapeutixTENS&PMScanhelppreventelectromagneticinterferencebymaintaininga minimum
distancebetweenportableandmobileRF communicationsequipment(transmitters)andtheTherapeutixTENS&PMSasrecommended
below,accordingto themaximumoutputpowerofthecommunications equipment.
Rated maximum output
power of transmitter
(W)
Separation distance according to frequency oftransmitter
(m)
150 KHz to 80 MHz
d 1.167 P
80 MHz to 800 MHz
d 1.167 P
800 MHz to 2.5 GHz
d 2.333 P
0.01
0.117
0.117
0.233
0.1
0.369
0.369
0.738
1
1.167
1.167
2.333
10
3.689
3.689
7.379
100
11.667
11.667
23.333
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating
of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply inall situations. Electromagnetic propagation is affected by absorption and
reflection fromstructures, objectsand people.
Symbol
Meaning
Class IIequipment
Type BFapplied part
Operatinginstructions
Manufacturer
Table of contents