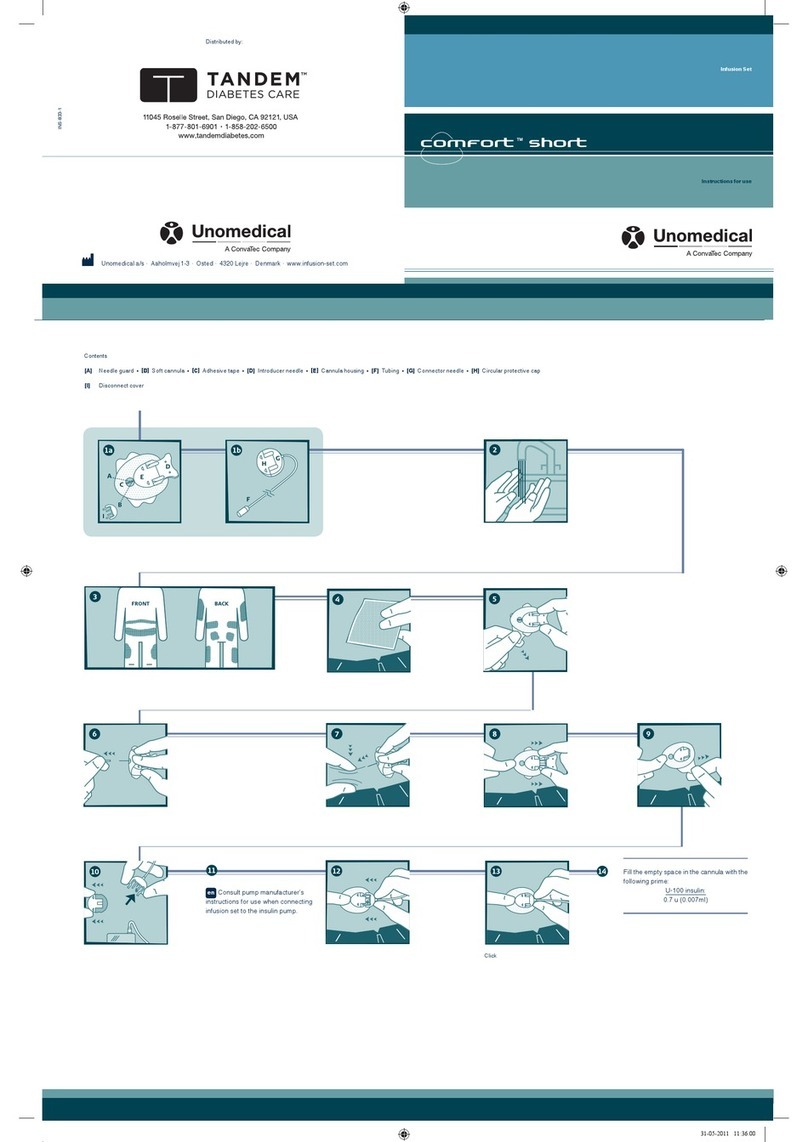
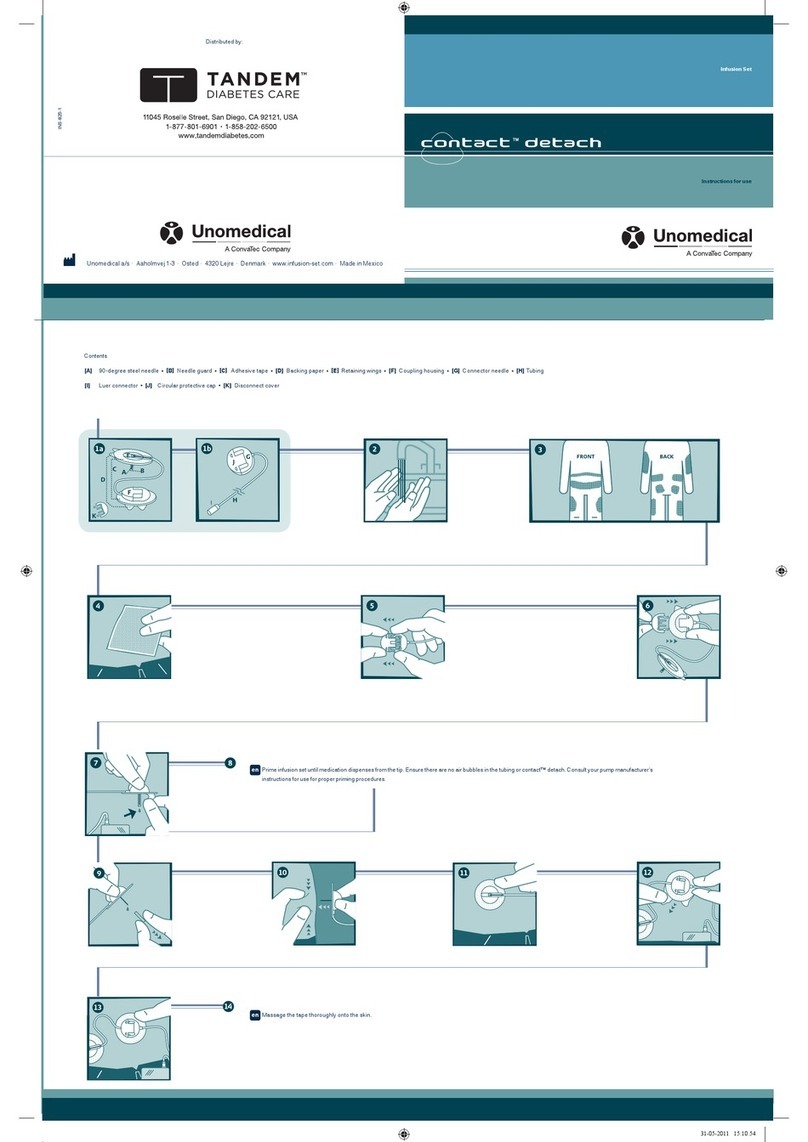
Click
E N G L I S H
Click
DISCONNECTING
RECONNECTING
Rx ONLY 0459
en Use by (year / month) en Do not re-use en Lot number en Sterilized using ethylene oxide en Reference / model number en Attention: see accompanying documents en Manufacturer
Indications for use:
The comfort™ infusion set is indicated for subcutaneous infusion
of medication, including insulin, administered by an external
pump.
CONTRAINDICATIONS comfort™ is neither intended
nor indicated for use with blood or blood products (i.v. infu-
sion).
WARNINGS
■
The comfort™ infusion set is only sterile and non-
pyrogenic if the packaging is unopened and undam-
aged. Do not use it if the packaging is already open
or is damaged.
■
Go through instructions for use carefully before inser-
ting comfort™, as failure to follow instructions may
result in pain or injury.
■
Inaccurate medication delivery, infection and/or site irri-
tation may result from improper insertion and maintenance
of infusion site.
■
When using comfort™ for the first time, do so in the pres-
ence of a healthcare provider.
■
comfort™ is a single use item, which must be disposed of
after usage. Do not clean or re-sterilise.
■
Be sure that the needle guard is removed before insertion.
■
People with small or large amounts of subcutaneous fat
should choose the insertion angle carefully, as the can-
nula could be placed in underlying muscle of dermal
layer and hereby cause reduced or blocked medication
delivery. Normal insertion angle between 20-45°. Consult
your healthcare provider concerning this matter.
■
Do not leave air in the infusion set. Make sure to fill
comfort™ completely.
■
Do not in any way put disinfectants, perfumes, deodorants
or other products containing alcohol or desinfectants in
contact with the luer-lock connector and the tubing, as
these may affect the integrity of the infusion set.
■
Replace the infusion set if the adhesive tape becomes
loose.
■
Check the infusion set frequently to ensure that the soft
cannula remains firmly in place, and replace if it is not in
place. Since the cannula is soft, it will not cause any pain
if the soft cannula becomes fully or partially dislodged
from the skin, and this may therefore take place without
notice. The soft cannula must always be completely
inserted to receive full amount of medication.
■
Replace infusion set every 48-72 hours according to
Centers for Disease Control guidelines, or per healthcare
provider’s instructions.
■
If infusion site becomes inflamed, replace set and use new
site until the first site has healed.
■ Do not re-inser t introducer needle into infusion set.
Re-insertion could cause tearing of soft cannula which
would result in unpredictable medication flow.
■
Never prime the infusion set or attempt to free a clogged
tubing while set is inserted. You may accidentally inject too
much medication.
■
Use aseptic techniques when temporarily disconnecting
comfort™, and seal the cannula housing with the cover
provided. Consult with healthcare provider on how to
compensate for missed medication when disconnected.
■
Protect comfort™ from direct sunlight. Store at room
temperature.
■ Reuse of the infusion set may cause infection, site irrita-
tion, or damage to the cannula/needle. A damaged can-
nula/needle may lead to inaccurate medication delivery
■
If infusing insulin, carefully monitor blood glucose levels
when disconnected and after reconnecting.
■
If infusing insulin, and blood glucose level becomes
unexplainably high, or occlusion alarm occurs, check for
clogs and leaks. If in doubt, change infusion set since soft
cannula could be dislodged, crimped or partially clogged.
Discuss plan for rapid replacement of insulin with health
care provider should any of these problems arise. Test
blood glucose level to make sure problem is corrected.
■
Check the blood glucose level 1-2 hours after introducing
comfort™ and check the place of infusion several times a
day, and measure your blood glucose regularly.
■
If infusing insulin, do not change infusion set just prior to
bedtime, unless blood glucose can be checked 1-2 hours
after insertion.
INS_830-1.indd 2 31-05-2011 11:22:01