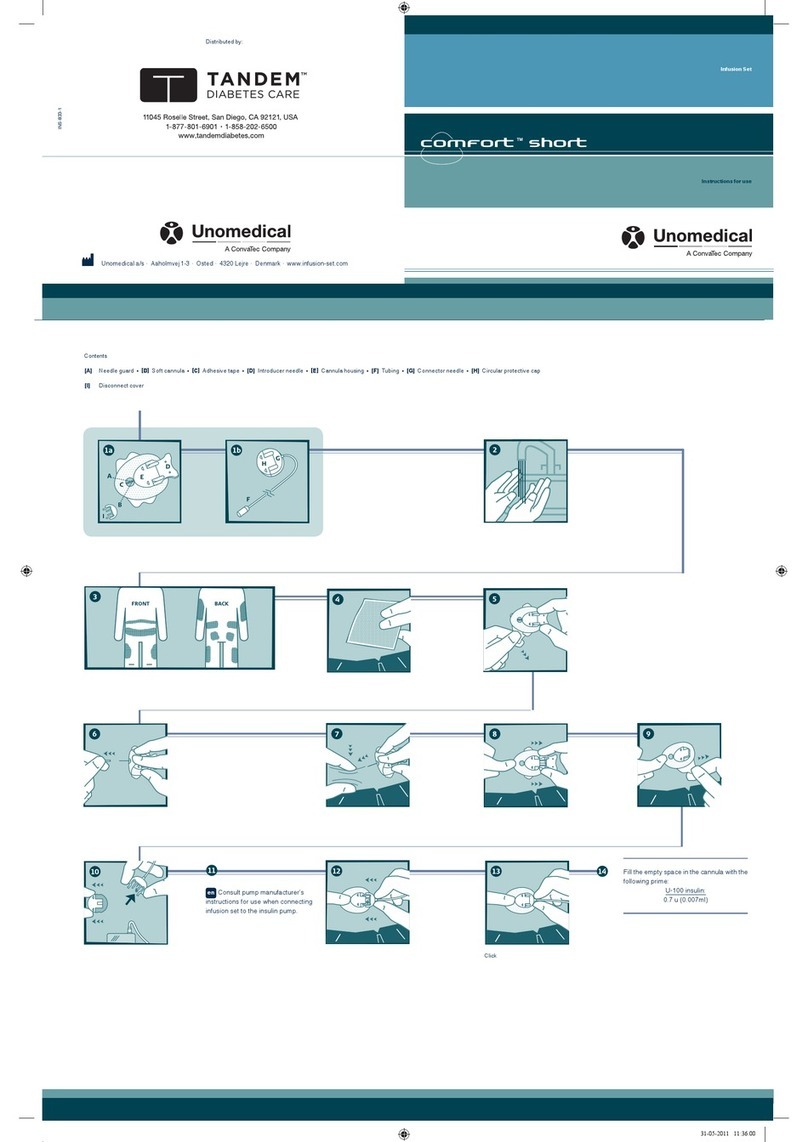
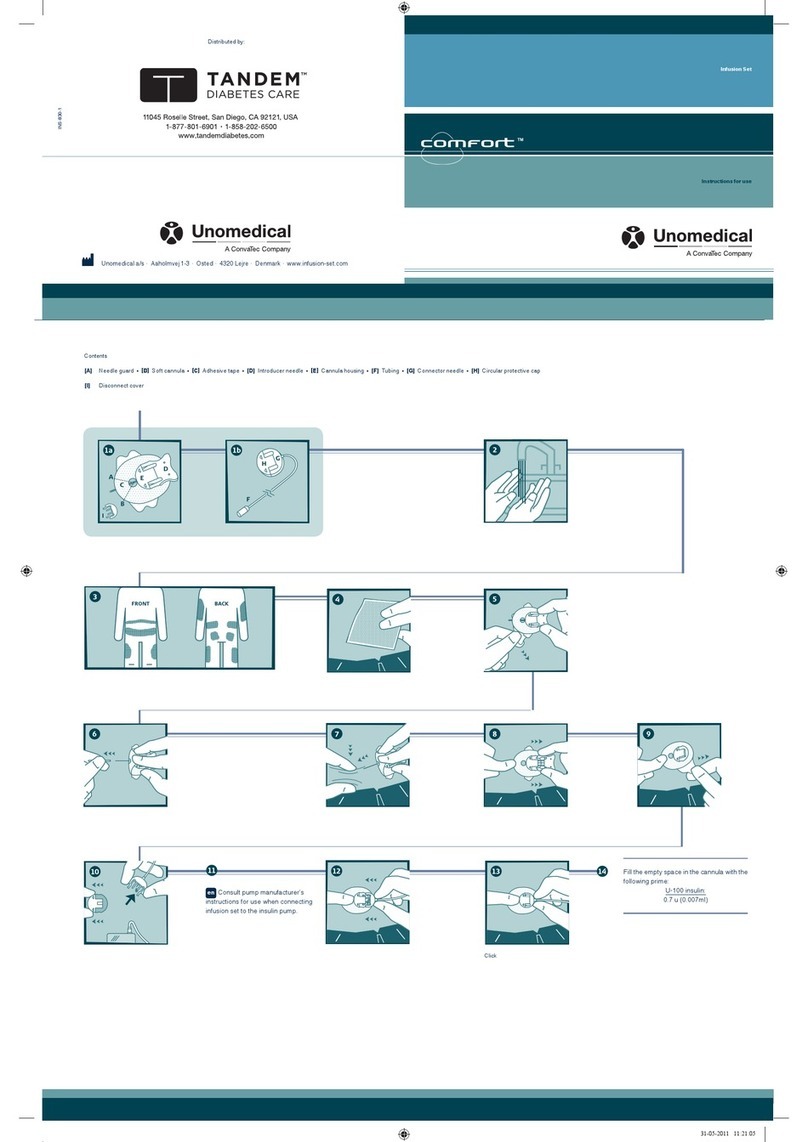
Click
E N G L I S H
INDICATIONS AND USAGE:
The contact™ detach is indicated for subcutaneous infusion of
medication, including insulin, administered by an external pump.
PRODUCT DESCRIPTION
contact™ detach is a 90 degree, stainless steel needle infusion
set with a standard luer lock connection.
CONTRAINDICATIONS
contact™ detach is neither intended nor indicated for use with
blood, blood products or intravenous infusion (I.V.).
WARNINGS
■ The contact™ detach infusion set is only sterile and
non-pyrogenic if the packaging is unopened and
undamaged. Do not use it if the packaging is already
open or is damaged.
■ Go through instructions for use carefully before inserting
contact™ detach, as failure to follow instructions may
result in pain or injury.
■ Inaccurate medication delivery, infection and/or site
irritation may result from improper insertion and
maintenance of infusion site.
■ When using contact™ detach for the rst time, do so
in the presence of a healthcare provider.
■ Reuse of the infusion set may cause infection, site
irritation, or damage to the cannula/needle. A damaged
cannula/needle may lead to inaccurate medication
delivery.
PRECAUTIONS
■Never prime the infusion set or attempt to free a clogged
tubing while the infusion set is inserted. You may accidentally
inject too much medication.
■Check the infusion set frequently to ensure that the steel
needle remains rmly in place. If the steel needle is not in
place, change to a new infusion set.
■contact™ detach is a single use item which must be dis-
posed of after usage. For available sharps disposal con-
tainers, please consult your local pharmacy.
■Do not clean or re-sterilize.
■Be sure that the needle guard is removed before insertion.
■The contact™ detach needle must not be bent prior to
insertion.
■ Do not leave air in the infusion set. Make sure to ll contact™
detach completely.
■Do not in any way put disinfectants, perfumes, deodorants or
other products containing alcohol or disinfectants in contact
with the luer lock connector and the tubing, as these may
aect the integrity of the infusion set.
■Replace with a new infusion set if the adhesive tape becomes
loose.
■Change the infusion set every 24-48 hours, or as recom-
mended by your healthcare provider.
■Dispose of used contact™ detach safely, so that no one can
prick or injure themselves with it.
■ If infusion site becomes inamed, replace with a new infusion
set and use new site until the rst site has healed.
■Use aseptic techniques when temporarily disconnecting
contact™ detach, and seal the coupling housing with the
cover provided. Consult with healthcare provider on how to
compensate for missed medication when disconnected.
■Protect the contact™ detach from direct sunlight. Store at
room temperature.
RECOMMENDATIONS
For use with insulin:
■ If blood glucose level becomes unexplainably high, or
occlusion alarm occurs, check for clogs and leaks. If in
doubt, change infusion set. Discuss plan for rapid re-
placement of insulin with healthcare provider should any
of these problems arise. Test blood glucose level to make
sure problem is corrected.
■ Check the blood glucose level 1-2 hours after introducing
contact™ detach and check the infusion site several times a
day, and measure your blood glucose regularly.
■ Do not change infusion set just prior to bedtime, unless blood
glucose can be checked 1-2 hours after insertion.
■ Carefully monitor blood glucose levels while disconnected
and after reconnecting
Click
DISCONNECTING
RECONNECTING
Rx ONLY 0459
en Use by (year / month) en Do not re-use en Lot number en Sterilized using ethylene oxide en Reference / model number en Attention: see accompanying documents en Manufacturer
INS_825-1contactdetach_Tandem.indd 2 31-05-2011 15:12:56