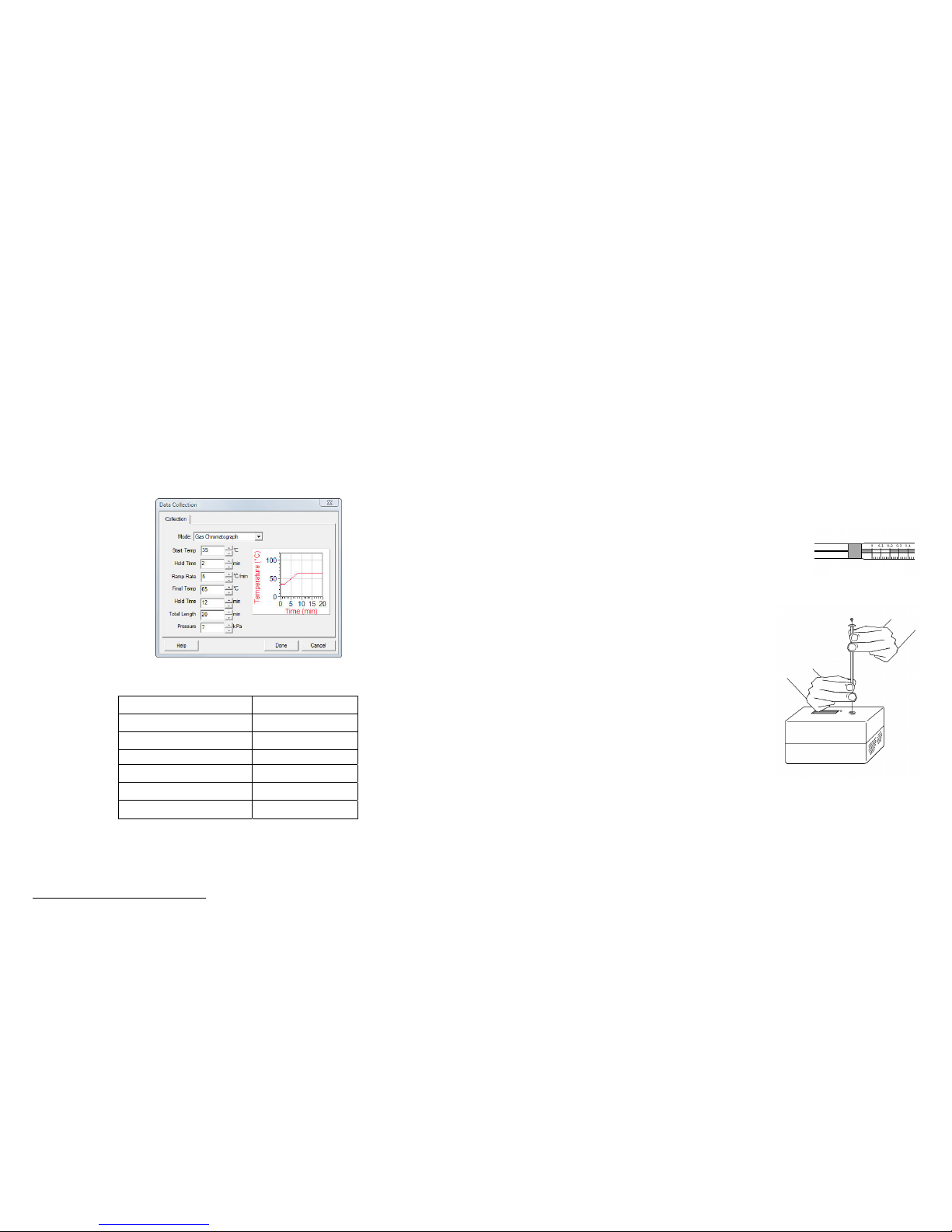
11
Appendix B
To comply with certification requirements for this instrument, the manufacturer
provides the following risk assessment:
Leakage: There is no risk from reagent leaks when using this instrument as
intended.
Fire/Flammability: To minimize risk of fire, the exhaust from the chromatograph is
vented at the rear panel of the instrument. In the unlikely event that a large injection
(1.0 μL) of a very flammable material such as hexane was to ignite by an ignition
source placed near the sensor outlet barb, the reaction would produce 3.5 calories of
energy. This energy is enough to raise the temperature of a gram of water 3.5°C or to
raise the temperature of the chromatograph (1.3 kg mass) by approximately
0.0035°C. This is very small when compared against roughly 252 calories released
from ignition of one blue tip match.
Electrical shock: To minimize the hazard of electrical shock, the chromatograph is
powered at 24 V. Use only the power supply provided by the manufacturer to power
this instrument.
Appendix C
Syringe Usage Instructions (1.0 L GC Syring)
Syringe Handling
1. Never pull the plunger back more than 80% of its total volume. Warning:
Once the plunger has been pulled out, it is almost impossible reinsert. You may
want to err on the side of caution, and never have students pull the plunger
back more than 50% (0.5 L). This avoids having students accidentally pull the
plunger from the syringe body, a costly mistake.
2. Exercise caution when depressing the plunger. Stickier chemicals can jam the
syringe and depressing the plunger too rapidly can cause the plunger to bend.
In the case of our 1.0 L syringe, the plunger is a fine wire that extends into the
needle. A bend in the plunger of this syringe may not be visible, but the
plunger will begin to stick which can render the syringe useless.
3. If the plunger starts to catch in the syringe during sample collection or
injection, follow the procedure for cleaning the syringe. NEVER force the
plunger.
4. If the syringe becomes clogged, do not pump the plunger or attempt to force
liquid or compressed air through it. The excessive pressure could cause the
barrel to crack.
5. Avoid unnecessary movement of the plunger in a dry syringe.
6. The plunger should only be grasped by the button since any abrasions,
scratches, or oil from one's fingers can interfere with proper plunger operation.
7. Always proceed carefully when inserting the syringe needle into the gas
chromatograph. Occasionally, the needle will catch on the column; inserting
the needle with too much force will cause it to bend, which will destroy the
syringe. Rotating the syringe will alleviate this problem.
12
8. When wiping the needle of the syringe, pinch a lint-free wipe around the
needle next to the barrel of the syringe and wipe in a single motion toward the
point of the needle. Never wipe the needle toward the barrel.
9. Syringes should be regularly inspected for damage, including hairline cracks.
Be sure to check the needle tip for barbs, which tear the septum, and can
produce particles that clog the needle or the chromatograph’s column. Cracked
syringes should be discarded according to your sharps disposal procedure.
Needle burrs can be smoothed with a fine emery board or carborundum.
10. Before storing a syringe, rinse it with acetone. Wipe dry all external surfaces.
Store the syringe in original shipping container or shock-absorbing padding.
11. NEVER soak the syringe for an extended period in a solvent. Doing so could
cause any adhesives used in the construction of the syringe to dissolve.
Tips
1. Greatest analytical accuracy is achieved when the injection volume is at least
20% of the working volume of the syringe. If the optimal syringe size is
unavailable, be aware that your results will show greater variability.
2. Prior to injection, moving the syringe plunger as slowly as possible during all
steps (whether liquid is involved or not) will help maintain the accuracy of the
injection volume.
3. When collecting your sample, grip the syringe by the flange and plunger button
only, as your body heat can affect the volume collected.
4. During insertion of the syringe needle into the gas chromatograph, support the
needle with one hand and hold the flange of the syringe in the other. This
reduces the chance of bending the needle.
5. If the needle seems to catch when inserting it into the gas chromatograph, turn
the syringe a quarter turn and then try again.
6. When injecting into the GC, you want to depress the plunger quickly enough to
ensure that the entire sample is delivered at once but carefully enough that you
do not bend the plunger if the plunger sticks. The larger injection sizes require
greater care during the injection.
Rinsing the Syringe
1. Submerge the tip of the syringe needle in the rinse liquid, commonly acetone
or ethanol, and draw the plunger back to 50% of its total volume.
2. Remove the syringe from the rinse liquid and place the tip of the needle over
an appropriate waste receptacle such as a beaker or lint-free tissue.
3. Slowly depress the plunger, ejecting all of the solution from the syringe.
4. Wipe the needle of the syringe with a fresh lint-free tissue.
5. Repeat the above steps 3 times.
6. Remove the syringe tip from the solution, replace the cap of the vial of rinse
liquid, and wipe the needle of the syringe with a fresh lint-free tissue.