CE-C10-03-SMSP01-20220502 V1.0
Contents
Introduce........................................................................................................................................ I
Maintenance and repair service.............................................................................................III
Intellectual Property Statement..............................................................................................III
1Safety precautions.....................................................................................................................1
1.1 Security classification .....................................................................................................1
1.2 Security symbol...............................................................................................................1
1.3 Safety warning information.............................................................................................2
1.4 WARNING Labels..........................................................................................................4
1.5 Ultrasound Benefits and Risks........................................................................................4
Ultrasound Benefits...........................................................................................................4
Ultrasound Risks...............................................................................................................4
2 Product overview ...........................................................................................................................5
2.1 Intended use ........................................................................................................................5
2.2 Contraindication..................................................................................................................5
2.3 Product specifications .........................................................................................................6
2.3.1 Imaging mode........................................................................................................6
2.3.2 Power condition ....................................................................................................6
2.3.3 environment condition...........................................................................................6
2.4 system configuration........................................................................................................8
2.4.1 Standard configuration..........................................................................................8
2.4.2 Components...........................................................................................................8
Transducer Type................................................................................................................8
2.5 Symbol description...........................................................................................................9
2.6 Introduction of each component of the system ..............................................................12
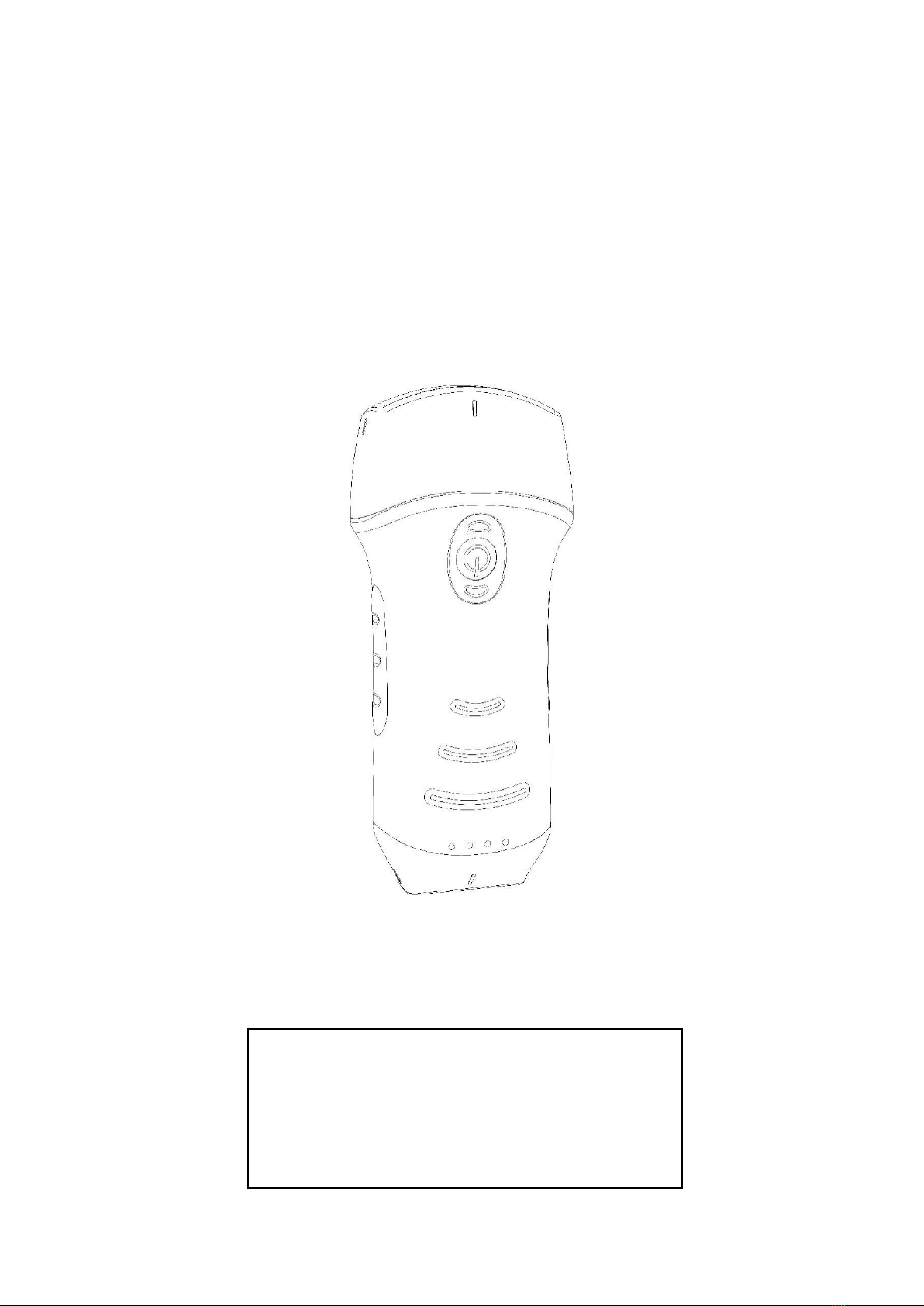
2.7 Control panel..................................................................................................................13
3 Basic introduction........................................................................................................................14
3.1 Install software...............................................................................................................14
3.1.1 iPhone/iPad .........................................................................................................14
3.1.2 Android device....................................................................................................15
3.1.3 Windows device..................................................................................................15
3.2 Turn on/off the probe .....................................................................................................16
3.3 Probe and Terminal connection......................................................................................17
3.4 Basic software interface.................................................................................................21
Convex array + Phased array mode: .......................................................................................21
4 Detailed operation introduction.................................................................................................22
4.1 Introduction to all levels of menu ..................................................................................22
4.1.1 First level menu...................................................................................................22
4.1.2 Introduction to the second level menu ................................................................22
4.2 Operation Introduction...................................................................................................23
4.2.1 B mode................................................................................................................23
Selecting Exam Present...................................................................................................26
Switching Between Imaging Modes ...............................................................................27