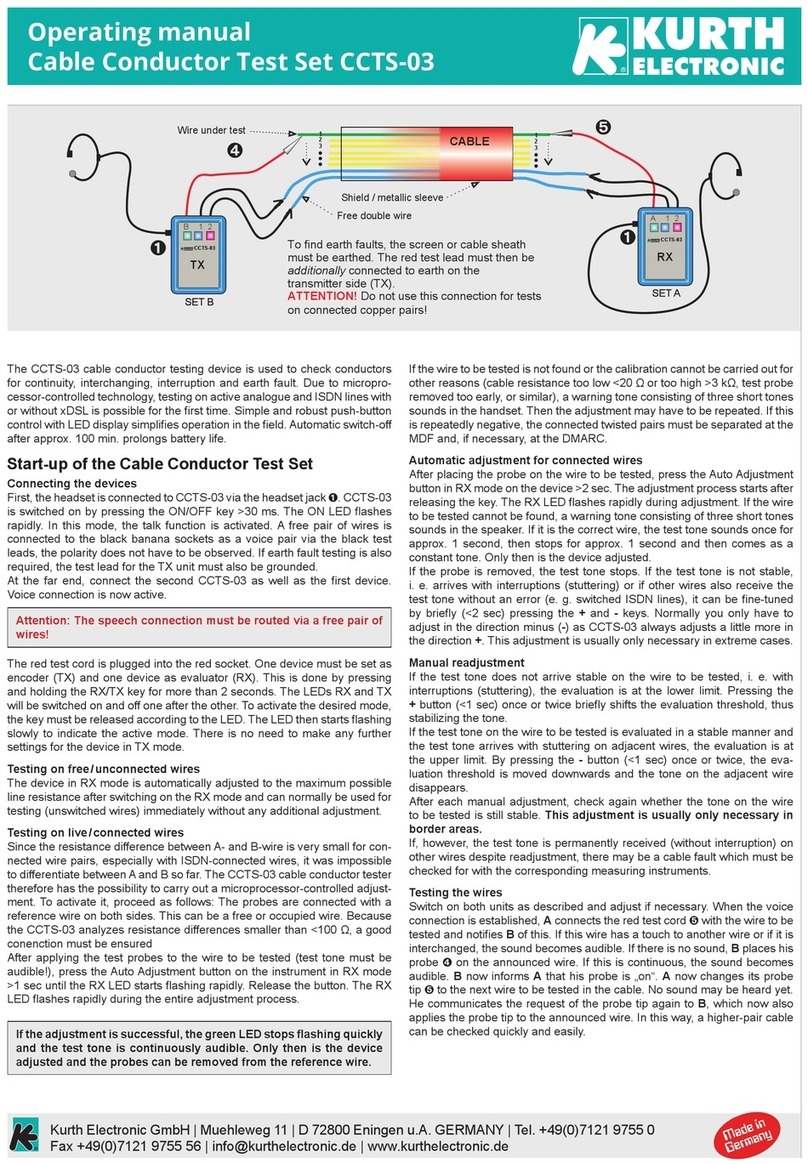
SC-300 manual 10 Version 2.1
3. Place 25 mL of wine or must in the titration vessel. We recommend using the 25 mL sampling
pipette provided in the kit: draw the sample up to the 0 mL mark, and then dispense the sample
into your titration vessel by letting the tip of the pipette touch the side of the vessel while the
sample drains (See Figure 6). NEVER pipette any reagents by mouth! Also make sure the
pipette you are using is completely clean before submerging into your wine sample.
4. Using the transfer pipettes (see Figure 7), add about 2 mL Acid Reagent and 2 mL Reactant
solution to the titration beaker (the two transfer pipettes should come with red and yellow labels,
with red for the Acid Reagent and yellow for the Reactant). It is not necessary to be extremely
accurate in this step; with these pipettes, 2 mL is roughly the amount that fills the pipette up to
the 2 mL mark after a single thorough squeeze of the bulb. To preserve the shelf life of these
reagents take care not to cross contaminate the transfer pipettes. If they do get contaminated
rinse them off with distilled water and let air dry. Caution: the Acid reagent is corrosive and
can cause damage to clothing, skin and eyes. The Reactant solution should not be ingested.
ALWAYS use safety glasses! We recommend the use of laboratory latex or nitrile gloves
during this procedure. If any solutions contact skin or eyes, flush with plenty of water.
5. If you are using a magnetic stirrer, turn it on to stir at a moderate rate. The Magnetic Stirrer
provided with the SC-300 Pro Kit operates at a suitable preset speed. Make sure your electrode
is not struck by the spinning stir bar. To prevent this, we recommend using the Vinmetrica
Electrode Holder to stabilize your electrode.
6. Rinse the electrode briefly with distilled water. Insert the electrode into the titration beaker so
that the tip is completely submerged to just above the circulation gaps (cutouts at the tip of the
electrode) but above the level of the stir bar (approximately half an inch from the bottom of the
titration beaker). If you are using the Vinmetrica Electrode Holder adjust it to a similar level.
7. If you are stirring manually, begin now; use a constant moderate swirling motion. If the
electrode is not held in a stand, hold it against the side of the vessel with one finger and grasp
the vessel with the remaining fingers so that the two move together. (See Figure 8).
8. Verify that the current is less than 50 and the green (“PROCEED”) LED is lit (See Figure 10).
If the current is greater than this, and/or the red (“STOP”) LED is lit and the buzzer sounds,
your sample has less than 2 ppm SO2and there is no need to proceed. (Verify that your SO2has
been preconditioned; see step 3 in the Instrument Operation section of the manual).
9. Titrate the sample by adding the SO2Titrant drop wise from the syringe (See Figure 8) or from
the burette (See Figure 9), being sure to note the starting volume mark on the syringe or burette.
Try to accomplish the titration as rapidly as possible (in 3 minutes or less), but be careful near
the endpoint so as not to overrun it –here, dispense one or two drops at a time. Be sure to
maintain stirring or swirling throughout the entire procedure. If the magnetic stirrer turns off,
turn it back on.