Vitalograph asma-1 USB User manual
asma-1 USB
Instructions for Use
MODEL 4000
© Copyright Vitalograph 2020 Current Edition (Issue 1, 28-Jan-2020) Cat. No. 09720

Vitalograph Ltd, UK
Maids Moreton, Buckingham
MK18 1SW
England
Tel: 01280 827110
Fax: 01280 823302
E-mail: [email protected]
www.vitalograph.co.uk
Technical Support
Tel: 01280 827177
Email: [email protected]
Vitalograph Ltd, International
Maids Moreton, Buckingham
MK18 1SW
England
Tel: +44 1280 827120
Fax: +44 1280 823302
E-mail: [email protected]
www.vitalograph.eu
Technical Support
Tel: +353 65 6864111
Email: [email protected]
Vitalograph GmbH
Rellinger Straße 64a
D-20257 Hamburg
Germany
Tel: +49 40 547391-40
Fax: +49 40 547391-40
www.vitalograph.de
Technical Support
Telefon: +49 40 547391-14
E-mail: [email protected]
Vitalograph Inc.
13310 West 99th Street
Lenexa, Kansas, 66215
USA
Toll Free: 800 255 6626
Tel: (913) 730 3200
Fax: (913) 730 3232
E-mail: [email protected]
www.vitalograph.com
Technical Support
Tel: (913) 730-3205
Email: [email protected]
Vitalograph (Ireland) Ltd
Gort Road Business Park
Ennis, Co Clare, V95 HFT4
Ireland
Tel: +353 65 6864100
Fax: +353 65 6829289
E-mail: [email protected]
www.vitalograph.ie
Technical Support
Tel: +353 65 6864111
Email: [email protected]
Vitalograph Ltd, Hong Kong/China
P.O. Box 812
Shatin Central Post Oce
Hong Kong
E-mail: [email protected]
www.vitalograph.cn
Technical Support
Tel: +353 65 6864111
Email: [email protected]
© Copyright Vitalograph 2020
Current Edition (Issue 1, 28-Jan-2020)
Cat. No. 09720
Vitalograph is a registered trademark.
Vitalograph Branch Addresses
DT_0006 Issue 15
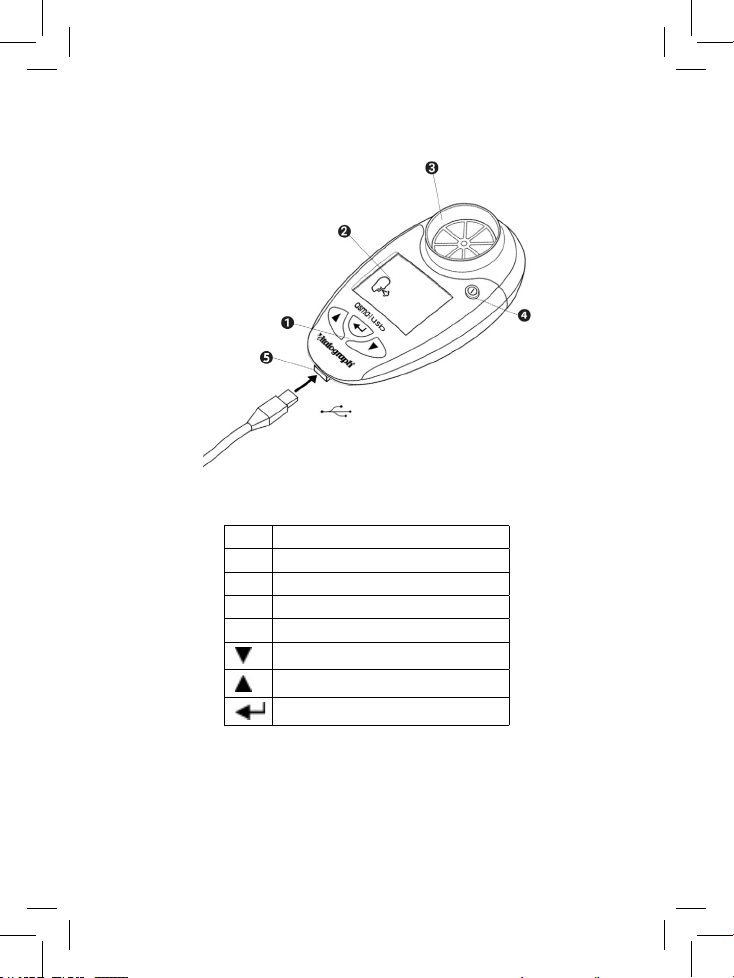
1. Main Components of the Vitalograph asma-1 USB
Figure 1 Vitalograph asma-1 USB Components
1 User Buttons
2 Display
3 Flowhead
4 On / Off button
5 USB
Down Button
Up Button
Enter Button

1.1. Features of the Vitalograph asma-1 USB
• Electronic records
• Stores 600 test sessions
• Download test results via USB and export to PDF report
using Vitalograph Reports software.
• Automatically assesses test quality
• Measures PEF and FEV1 and % of personal best
• PEF and FEV1 zones can be personalized
• Automatically stores best values
2. Setting Up the Vitalograph asma-1 USB
To get the Vitalograph asma-1 USB ready for use:
1. Remove the detachable battery door at rear of unit. Fit two
AAA 1.5V batteries. Replace battery door.
2. Attach a mouthpiece to the owhead.
Figure 2. Mouthpiece inserted into owhead

3. If used for multiple subjects, Vitalograph intends that a
new Eco Bacterial Viral Filter (Eco BVF) be used for every
test subject to prevent cross contamination. Using a new
Eco BVF provides a signicant level of protection of the
subject, the device and the user against the risk of cross
contamination during spirometry manoeuvres. Eco BVF
and SafeTway mouthpieces are single use items and must
be disposed of after use.
4. Turn on via the On/Off Button. (The same button is used
to power down.)
3. Operating Instructions
If the device has just been unpacked or transported, ensure that it is
left sitting, fully powered and is at room temperature prior to testing.
3.1. Setting Personal Best (Reference) Values
Personal Best (reference) values can be set for peak ow (PEF)
and/or forced expiratory volume after 1 second (FEV1).
To set the Personal Best (reference) PEFs:
1. Turn the device on, .
2. When the device is ready for a test ( ), press the and
buttons together for 3 seconds.
3. Set the reference PEF value by pressing the button and
releasing when the value is reached. Press the to roll
back. The values will increase/decrease in values of 10. If
the button is kept depressed, the values will scroll faster.
4. Press ENTER to save the reference PEF value.
5. Press to exit, OR to set the Best Test (reference)
FEV1:
6. Set the reference FEV1 value by pressing the button and
releasing when the value is reached. Press the to roll
back. The values will increase/decrease in values of 0.10.
If the button is kept depressed, the values will scroll faster.
7. Press ENTER to save the reference FEV1 value. The
device will return to the test screen.
Note: to de-activate zones, set both the PEF and FEV1 reference
values to 000/0.00.

3.2. Setting Management Zones
The asma-1 USB can be set for use with 3 or 4 zone management
plans. The zones are factory set to 2 boundaries (80% & 50%)
resulting in 3 Zones (0-50%, 50-80%, 80-100%). For 4 zones the
middle boundary is set last. The colour systems for each zone are:
Top Boundary
Green Green Top Boundary
Yellow Yellow
Orange Middle Boundary
Bottom Boundary Red Red Bottom Boundary
3 Zone 4 Zone
To set boundary percentage values for 3 zones:
1. Turn the device on, .
2. When the device is ready for a test ( ), press the and
buttons together for approximately 3 seconds.
3. Set the top (Green/Yellow) boundary by pressing the
or button and releasing when the value is reached. The
values will increase/decrease in values of 1%. If the button
is kept depressed, the values will scroll faster.
4. Press ENTER to save the boundary value.
5. Set the bottom (Yellow/Red) boundary by pressing the
or button and releasing when the value is reached.
6. Press to save the boundary value.
7. Select the next value as 0% (default). Only 2 boundaries are
required for the 3 zone system.
8. Press . The device will return to the test screen.
To set the boundary percentage values for 4 zones:
1. Set the top and bottom boundaries – see steps 1 – 6
above.
2. Set the middle (Yellow/Orange) boundary by pressing the
button and releasing when the middle boundary value
is reached. The values will increase/decrease in values of
1% after an initial jump to the lower boundary value. If the
button is kept depressed, the values will scroll faster. This

boundary value cannot be set at a value that is greater than
the top boundary value or less than the bottom boundary
value.
3. Press to save the middle (Yellow/Orange) boundary
value. The device will return to the test screen.
3.3. Performing a Test
1. Fit a mouthpiece, SafeTway or Eco BVF lter to the device.
2. Turn the device on.
3. Sit down to blow into the device (unless the physician
advises otherwise).
4. When the device is ready for a test ( ), holding the head
high, breathe in as deeply as possible, hold the Vitalograph
asma-1 USB ready in front of the mouth, see Figure 3.
Figure 3. Holding device during test.
5. Holding the breath, place the mouthpiece into the mouth,
biting the mouthpiece lightly, and with the lips rmly sealed
around it.
6. Blow out as HARD and as FAST as possible for a second
or more. Be careful not to block the mouthpiece with the
tongue or teeth. A ‘spitting’ action will give false readings.
7. The PEF result for this blow displays on screen, followed
by the FEV1 result after approximately 3 seconds. If the
Personal Best has been set, an arrow indicates which
colour management zone the blow relates to.
8. When the blow icon shows, blow again ( ). Usually 3
blows are required.
9. To view the best test in the session (best PEF and best
FEV1), press the button. This value is recorded as the
result for the session in the device history.
Note: An exclamation mark ! indicates a poor quality blow and the
blow should be repeated.
The ! warning appears when the time to Peak Flow > 120ms or a
cough is detected in the rst second.
If dizziness or fatigue is experienced during the test session, wait
until this passes before blowing again, or terminate the session.
3.4. Reviewing Previous Results
The asma-1 USB can store up to 600 test sessions. To view
previously performed test sessions:
1. When the device is ready for a test ( ), press the
button for approximately 3 seconds.
2. The most recent test session displays. The best PEF result
will be displayed for approximately 3 seconds, followed
by the best FEV1 result. The session number ‘1’ is also
displayed, this is the latest session.
3. View earlier test sessions by pressing the button.
4. Press . The device will return to the test screen.
3.5. Deleting All Results History
Caution: Results history cannot be recovered once it has been
deleted.
To delete entire history, i.e. all previously stored session results:
1. When the device is ready for a test ( ), press the and
buttons simultaneously for approximately 10 seconds.
2. A long beep indicates success. The device returns to the
test screen.
3.6. Downloading saved results
The test session may be transmitted to Vitalograph Reports
software on your PC where it can be stored as a PDF le and viewed
or printed.
Vitalograph Reports must be installed and running on the PC before
data is transmitted. The software is running if the Vitalograph ’V’

icon shows in the PC System Tray. If not, refer to help button of the
software for more information. The USB cable should be attached
to the device and also to the host PC to allow communication, see
Figure 4. Drivers should automatically install.
Figure 4. Attached USB cable.
1. Run Vitalograph Reports.
2. If the ‘No devices have been detected’ message appears,
click ‘Continue’.
1. Turn on Device via the On/Off Button
2. In Vitalograph Reports ensure ‘Vitalograph asma-1’
is selected as the device via the Device Selection
Button
3. To print the test results from the asma-1 USB:
1. When the device is ready for a test ( ), press the
button for 3 seconds. The device will show the
Report icon ( ). OR
2. After completing the test session, press the or
button until the Report icon ( ) is displayed.
Press .

4. Once the print icon is displayed on the device, start the
service as shown by selecting ‘Start Report Service’ from
the ‘File’ Menu
5. Vitalograph Reports will download the report and the report
will be generated.
6. In Vitalograph Reports enter the Subject Demographic
details and Comments. When the information is entered
select ‘Continue’.
7. The PDF report saves in the default location: C:\
Vitalograph\VitalPrint2.0\Reports
4. Power Management
The asma-1 USB operates with 2 AAA 1.5V disposable batteries.
If the battery symbol ashes the batteries should be replaced.
Replace the batteries by removing the battery door on the underside
of the device.
Note: Dispose of used batteries safely.
5. Cleaning & Hygiene
The Vitalograph asma-1 USB is not designed or supplied as a
‘sterile’ device. Keep the device clean and dust free. If you suspect
the device is damaged or is measuring incorrectly, contact your
medical professional immediately.
The Vitalograph asma-1 USB should continue to give reliable
measurements for up to three years in home use. It should then be
replaced with a new device.
5.1. Cleaning in Single Patient Environment
For single patient use, the plastic mouthpiece may be used.
Weekly cleaning of the mouthpiece, outside surfaces and owhead
of the device is recommended. A cloth impregnated with 70%
isopropyl alcohol may be used. The reusable plastic mouthpiece
may be washed in warm soapy water and then rinsed in clean water.
The device should be cleaned before and after an extended period
of storage.

5.2. Preventing Cross-Contamination of Subjects in Clinic use
For multi-patient use in a clinic or telemedicine environment
Vitalograph recommends the use of Eco BVF lters or, if these aren’t
available then SafeTway mouthpieces may be used based on the
customer own risk assessment and hygiene controls.
Before use by the next subject, the mouthpiece, outside surfaces
and owhead of the device should be cleaned with a cloth
impregnated with 70% isopropyl alcohol. The device should be
cleaned before and after an extended period of storage. If you
suspect that a device intended for multi patient use has become
contaminated, it should be replaced.
When used in the clinic environment, it is recommended that
the device be replaced annually. There is no planned preventive
maintenance for this medical device.
6. Fault Finding Guide
Problem Fault
Symptoms:
No ow measurements
Possible Causes:
(In probable order)
The batteries may be low. Replace the
batteries.
The owhead may be damaged. Check that
the rotating vane is spinning freely.
Problem Fault
Symptoms:
Cannot read user interface
Possible Causes: The batteries may be low. Replace the
batteries.
7. Customer Service
For further assistance, setting up, using or maintaining the device
or to report unexpected operations or changes in performance,
contact Vitalograph, using the contact information at the start of
this manual. Also contact the healthcare provider on any changes to
the performance of the device, as a precaution.
Service and repairs should be carried out only by the manufacturer,
or by Service Agents approved by Vitalograph. Contact information
for approved Vitalograph Service Agents may be found at the start
of this manual.
Any serious incident that has occurred in relation to the device
should be reported to Vitalograph or its Authorized Representative
and the Regulatory Authorities of the country. Refer to the
Vitalograph contact information at the start of this manual.
8. Consumables and Accessories
Cat. No Description
28501 Eco BVF (100)
28572 Eco BVF + Disposable Noseclips (80)
40168 Plastic Mouthpiece 4000 series (20)
20303 Disposable Noseclips (200)
40167 Pouch Spare (x10)
20242 SafeTway Mouthpieces (200)
40079 USB Cable - 1M MINI-B to USB A
9. Disposal
The device and USB cable must be taken to separate collection
at the product end-of-life. Do not dispose of these products as
unsorted municipal waste. The pouch can be disposed of in
unsorted municipal waste.
Used Eco BVFs and SafeTways, constitute minimally soiled waste
from human healthcare and should be disposed of in line with local
requirements. Eco BVFs are made from 100% polypropylene.

10. Explanation of Symbols
Symbol Description
Type BF equipment
VA Power rating
Direct current
Instructions for Use; operating instructions
Manufacturer
Date of Manufacture (include date in format yyyy-mm-dd)
The device must be taken to separate collection at the
product end-of-life. Do not dispose of these products
as unsorted municipal waste
Serial Number
Device Order Number
Use by Date (Date format yyyy-mm-dd)
Keep Dry
Do not re-use
Non sterile
Recycle

Symbol Description
QR code - matrix bar code. All information in the bar
code is included in the text under it
Other Labels
Battery status
Battery status Full
Battery status Half
Battery status Quarter
Battery status Empty (ashing)
Blow Now Symbol
!Bad Test Symbol
Memory 90% - 100% Full Icon
Flashes when memory reaches 100%
Transmit Report Symbol
11. Description of the Vitalograph asma-1 USB
The Vitalograph asma-1 USB is a medical device intended to give
objective measures of lung function for help in managing asthma.
It is intended to be used in a variety of professional healthcare
environments, e.g. primary care, hospitals and occupational health
centres as well as in the home. The device measures airow out of
the lungs when blown into as hard and fast as possible. The asma-1
USB can reveal narrowing of the airways well in advance of an
asthma attack being felt by the asthmatic. Used mainly by persons
with moderate to severe and persistent asthma, the asma-1 USB
can help determine:
• When to seek emergency medical care
• The effectiveness of an asthma management and treatment
plan
• When to stop or add medication, as directed by the physician

• What triggers the asthma attack (such as exercise-induced
asthma)
11.1. Indications for Use
The Vitalograph asma-1 USB is a hand held respiratory monitor
which measures subject respiratory parameters FEV1 and PEF. It
is designed for lung function testing of adults and children, 5 years
and older, in home and professional healthcare environments, e.g.
primary care, hospitals and occupational health centres. The device
is intended to be operated by the patient, under the supervision of a
healthcare provider.
12. Technical Specication
Product Respiratory Monitor asma-1 USB
Model 4000
Dimensions 109mm (length) x 63mm (width) x
42mm (height)
Weight 63g (not including batteries)
Flow Detection
Principal
Stator/rotor
Accuracy Better than ± 3% (FEV1), ± 10% (PEF)
Back pressure Less than 0.15kPa/L/second @ 14L/s
Measurement Range: PEF: 25 – 840 L/min BTPS
FEV1: 0 – 9.99 L BTPS
Maximum test
duration
1 second
! Bad Test Criteria Time to Peak Flow >120ms or a cough
detected in the rst second
Power Supply 3V (2 x 1.5V AAA batteries)
Battery Life 3 months of use, 3 tests per day
(Batteries near the end of their shelf life
will have reduced capacity.)

Product Life 6 Years for the main device. The Eco
BVF, SafeTway & nose clips are single
use.
Operating temperature
range
17–37ºC
Operating humidity
range
30%–75%
Ambient pressure
range
850hPa–1060hPa
Performance
standards:
ATS/ERS 2019, ISO 23747:2015, ISO
26782:2009
Safety standards EN60601-1, EN 60601-1-11
EMC Standards EN 60601-1-2
QA/GMP standards EN ISO 13485, FDA 21 CFR 820, CMDR
SOR/98-282, JPAL, MDSAP.
Communications USB2.0/3.0
PC Requirements Windows® 7, Windows® 8 or
Windows® 10
13. Contraindications, Warnings, Precautions and
Adverse Reactions
1. No modication of this equipment is allowed. Any
unauthorised changes to the device may compromise product
safety and/or data and as such Vitalograph cannot be held
responsible and the device will no longer be supported.
2. The device should only be used under the supervision of a
healthcare professional.
3. The device is not designed as a sterile device. Always follow
the safety guidelines given by the manufacturer of cleaning
and disinfectant chemicals.
4. If used for multiple subjects, Vitalograph intends that a new
Eco Bacterial Viral Filter (Eco BVF) be used for every subject to

prevent cross contamination. Using a new Eco BVF provides a
signicant level of protection of the subject, the device and the
user against the risk of cross contamination during spirometry
manoeuvres. An Eco BVF is for single use only.
5. Spirometry is a valuable tool that provides important
information to clinicians which is used together with other
physical ndings, symptoms, and history to reach a diagnosis
(ATS/ERS 2019). And as such, spirometry may support or
exclude diagnosis, but it cannot make one.
6. Take care not to block the mouthpiece with the tongue or
teeth during testing. A ‘spitting’ action or cough will give false
readings.
7. Subject fatigue may occur during testing depending on the
subject’s characteristics e.g. age, health status. For safety
reasons, testing should be preferably done in the sitting
position, using a chair with arms and without wheels. Subject
can also take a break between tests.
8. All values displayed are expressed as BTPS values.
9. Time zero is determined using the back-extrapolated method,
from the steepest part of the curve.
10. Symptoms must take precedence over device measurements.
If the patient at home thinks that the device is not reading
correctly, they must advise the healthcare professional
immediately.
11. Do not expose the device to liquids other than cleaning liquids
specied.
12. Keep device dry. If the device gets wet, do not use it, and
contact Vitalograph using the contact information at the start
of this manual. Do not connect any part of this device to mains
power as there is a risk of injury especially if the device is wet.
13. The device is not intended to be used in the presence of
ammable liquids or gases, dust, sand or any other chemical
substances.
14. Service and repairs should be carried out only by the
manufacturer or by Service Agents specically approved by
Vitalograph.
15. RF communications equipment (including peripherals such
as antenna cables and external antennas), which emit

electromagnetic elds, should be used no closer than 30 cm
(12 inches) to any part of the device, including cables specied
by Vitalograph. Otherwise, degradation of the performance of
this equipment could result.
16. The device is a Type BF applied part. The subject comes into
contact with the device, mouthpiece, SafeTway or Eco BVF
during use.
17. The USB cable is a potential strangulation hazard. Adult
supervision is required at all times, when a child is using the
device.
18. Take care during battery replacement. An AAA battery is a
potential choking hazard for a small child. Adult supervision
is required at all times, when a child is using the device. The
battery door, when removed, has pointed corners which may
present a risk of injury.
19. The batteries should be removed if the device is intended to be
stored or left unused for an extended period of time.
20. Only approved accessories from the manufacturer should be
used with the device. It is may be unsafe to use accessories,
detachable parts and materials not described in this document.
21. Non-Medical Electrical equipment used with the device, should
comply with its relevant IEC or ISO standard.
22. For the device to be used as intended, there is no requirement
to clean the supporting computer. If cleaning is required to
remove any visible soiling, this should be done as per the
computer manufacturer’s instructions.
14. CE Notice
Marking by the symbol indicates compliance of the Vitalograph
Model 4000 asma-1 to the Medical Devices Directive of the
European Community.
The Vitalograph Model 4000 asma-1 is intended for use in a variety
of Home and professional healthcare environments, e.g. primary
care, hospital wards and occupational health centres, except for
near active high frequency surgical equipment and the RF shielded
room of an ME system for magnetic resonance imaging, where the
intensity of electromagnetic disturbance is high. The customer or
the user of the asma-1 should assure that it is not used in such an
environment.
The Model 4000 asma-1 has been tested in accordance with:
EN60601-1:2006 + A1:2013
Medical electrical equipment. General requirements for basic safety
and essential performance
EN 60601-1-11: 2015
Medical electrical equipment. General requirements for basic safety
and essential performance. Collateral Standard: Requirements for
medical electrical equipment and medical electrical systems used
in the home healthcare environment.
EN 60601-1-2: 2015
Medical electrical equipment - Part 1-2: General requirements
for basic safety and essential performance - Collateral Standard:
Electromagnetic disturbances - Requirements and tests.
EN 60601-1-2 - Emissions tests
Emissions test Compliance Electromagnetic
environment - guidance
RF emissions
CISPR 11 Group 1
The Model 4000 asma-1
uses RF energy only for its
internal function. Therefore,
its RF emissions are very low
and are not likely to cause
any interference in nearby
electronic equipment.
RF emissions
CISPR 11 Class B
The Model 4000 asma-1
is suitable for use in all
establishments, including
domestic establishments.

EN 60601-1-2 Immunity tests
Immunity test Test level Compliance level
Reached
Electrostatic
discharge (ESD)
EN 61000-4-2
Contact: ± 8 kV
Air: ± 2 kV,± 4 kV,
± 8kV,± 15 kV
Contact: ± 8 kV
Air: ± 2 kV,± 4 kV,
± 8kV,± 15 kV
Radiated RF
EN 61000-4-3
3 V/m
80MHz to 2700MHz
3 V/m
80MHz to 2700 MHz
Proximity elds
from RF devices
EN 61000-4-3
9 to 28V/m
385 to 5785MHz
As per Table 9
EN60601-1-2:2015
9 to 28V/m
385 to 5785MHz
As per Table 9
EN60601-1-2:2015
Medical Devices may be affected by mobile RF communications
equipment including cellular telephones and other personal
or household devices not intended for medical facilities. It is
recommended that all equipment used near the Vitalograph product
comply with the medical electromagnetic compatibility standard
and to check before use that no interference is evident or possible.
If interference is suspected or possible, switching off the offending
device is the normal solution, as is required in aircraft and medical
facilities.
Medical electrical equipment needs special precautions regarding
EMC and needs to be installed and put into service according to the
EMC information provided.
15. FDA Notice
Caution: Federal Law restricts this device to sale by, or on the order
of a physician.
This manual suits for next models
2
Table of contents
Other Vitalograph Personal Care Product manuals