8 | Avid IF2 Patient Manual
ENGLISH
CAUTION:
DO NOT apply lotions, oils or other ointments to the skin prior to the application
of electrodes. Topical agents may increase the risk of skin irritation.
DO NOT clean the skin with anything other than soap and water. Other cleaning
agents may interfere with proper conduction.
USE OF electrodes smaller than 2” round or 2x2” square is not recommended
as increased power density could cause skin irritation or burn.
USE OF electrodes for long periods of time without removal may cause skin
irritation.
In the event of skin irritation, discontinue use immediately and contact VQ OrthoCare’s
Patient Care Department at 800.452.7993 or consult your physician or therapist.
Starting Your Treatment Sessions
1. Prepare the skin. Electrode application sites must be clean, dry, unbroken
skin surfaces. To reduce the risk of skin irritation, follow these instructions
for proper skin care before treatment.
^Wash site of electrode placement with mild soap and water before
applying electrodes.
^Rinse all soap o the skin before continuing.
^Dry the skin thoroughly.
^Trim excess body hair from electrode sites with scissors. Be
careful not to cut (or break) the skin. DO NOT SHAVE THE SKIN
IMMEDIATELY PRIOR TO STIMULATION. Shaving will create small
cuts in the surface layer of skin.
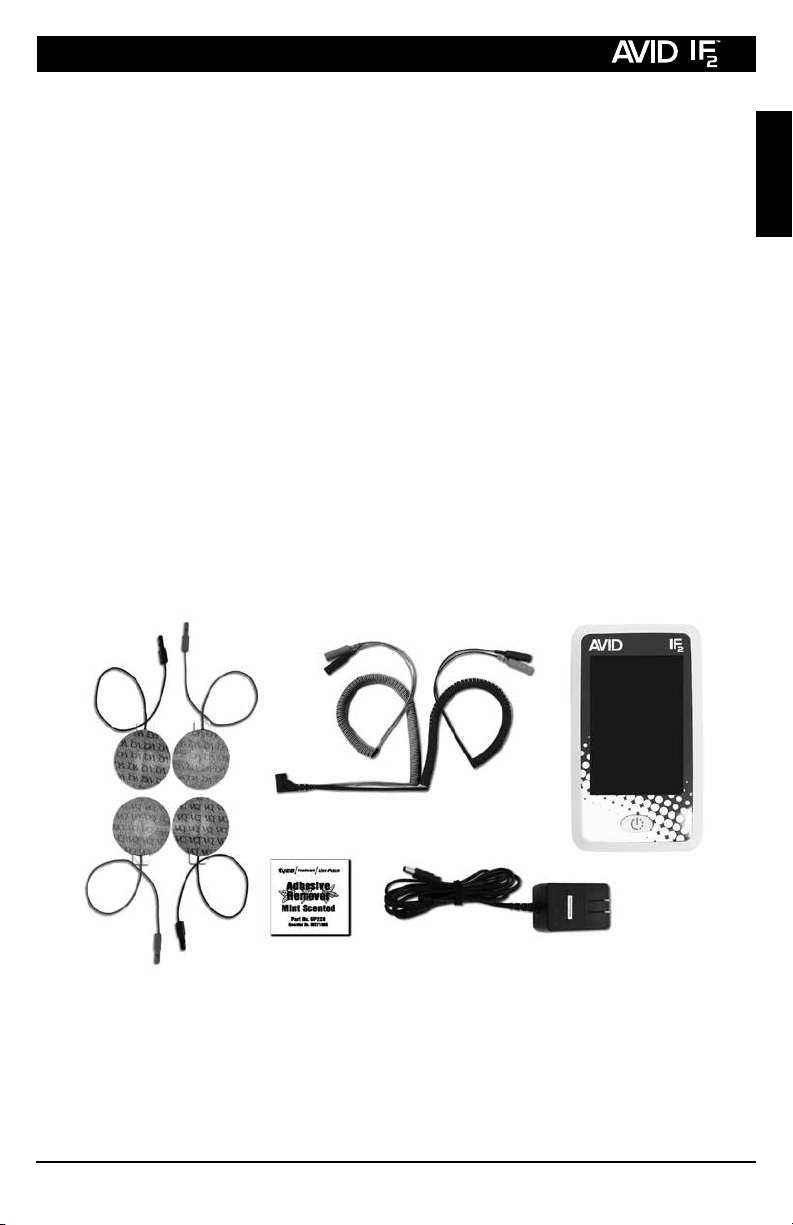
2. Inspect each electrode and lead wire before application. Do not
proceed if there is any doubt about the integrity or proper function of any
electrode or lead wire. Connect the lead wire to the electrodes. Make
sure there is no bare metal exposed at the connection points. Do not
use unnecessary force in connecting the electrodes to the lead wires. If
they do not fit, use a dierent set of electrodes and call VQ OrthoCare
to report the situation and receive a replacement set of electrodes.
Save the non-working electrodes for evaluation by VQ OrthoCare’s
Quality Assurance Department; DO NOT THROW THEM AWAY!
3. Gently remove the electrodes from the plastic liner. Place the electrodes
on the sites prescribed by your physician or therapist and press them
firmly onto the skin. Electrodes should be placed at least one inch
apart from each other. Skin irritation or burn could result.