VQ OrthoCare is not liable for misuse or misunderstanding of the BioniCare
product or operating manual. In the US, please call VQ OrthoCare’s Patient
Care Department at 800.444.1456 if any additional assistance is required
regarding this product and its operating instructions. Outside the US, please
contact your BioniCare System provider or your healthcare practitioner.
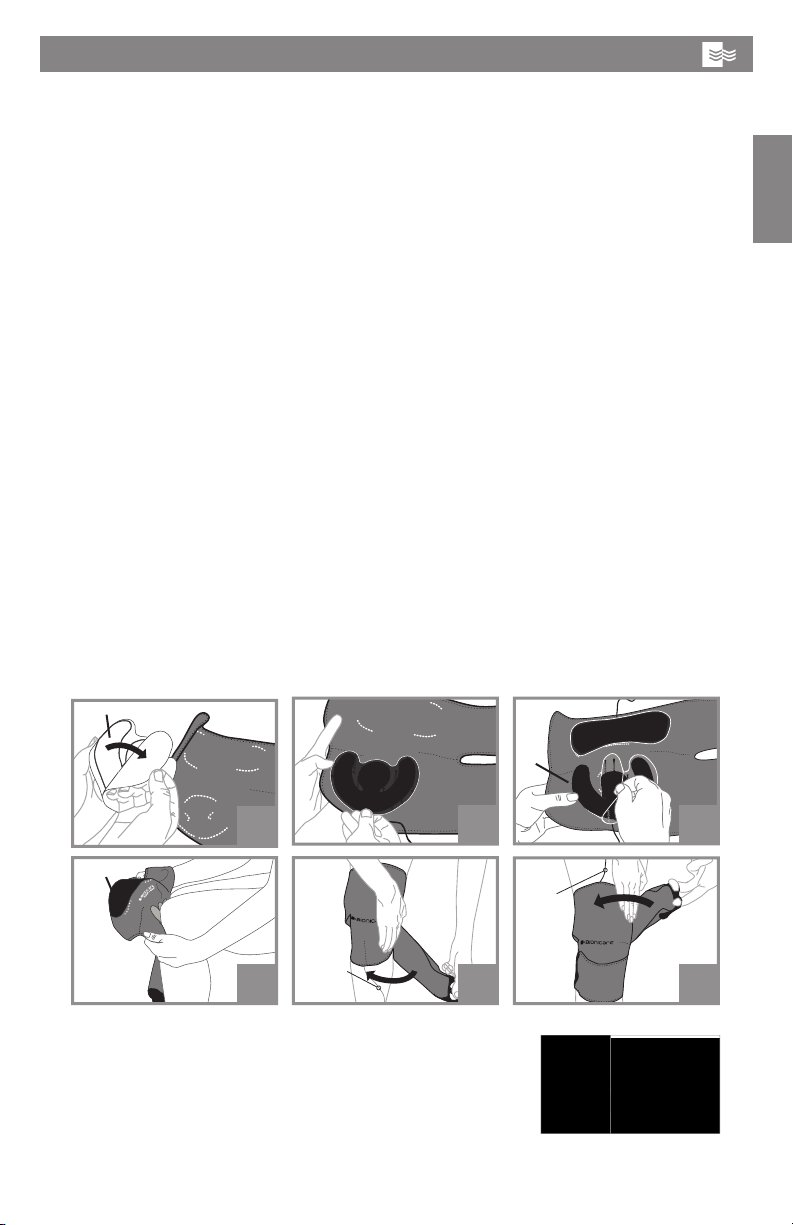
Operating Manual: BioniCare Knee System for the treatment of osteoarthritis
of the knee
US 5,273,033; CA 2,102,759; DE 69328429.3;
EP/IT 0652028; EP/GB 0652028; EP/FR 0652028;
EP/NL 0652028; EP/CH 0652028; EP/LI 0652028;
Other Patents Pending / Otras Patentes Pendientes / Autres Brevets en Instance
VQ OrthoCare no se hará responsable de ningún uso incorrecto o mala
interpretación del producto BioniCare o su manual de instrucciones. Para
obtener ayuda adicional sobre este producto y sus instrucciones de uso,
en los EE. UU. puede llamar al departamento de Atención al Paciente de
VQ OrthoCare al 800.444.1456. Fuera de los EE. UU., comuníquese con un
distribuidor del sistema BioniCare o con su profesional médico.
Manual de Instrucciones: Sistema para rodilla BioniCare para el tratamiento
de la osteoartritis de rodilla
VQ OrthoCare n’est en aucun cas responsable de la mauvaise utilisation
du produit BioniCare ou de tout malentendu concernant le contenu du
manuel d’utilisation. Si vous avez besoin de plus amples renseignements
concernant ce produit ou son utilisation et que vous résidez aux États-
Unis, veuillez appeler le Patient Care Department de VQ OrthoCare
au +1.949.261.3000. Si vous résidez en-dehors des États-Unis, veuillez
appeler le fournisseur de votre système BioniCare ou votre professionnel
de la santé.
Manuel d’utilisation: Système pour genou BioniCare pour le traitement de
l’arthrose du genou
ENGLISH
ESPAÑOL
FRANÇAIS