Lara| Instructions for Use |RIL-1 W&H Med ENG Rev06 |14/04/2022 |© W&H Sterilization Srl 9
Introduction
COPYRIGHT NOTICE
Copyright ©, W&H Sterilization Srl
All rights reserved in all countries.
All drawings, images and texts contained in this manual are the
property of the manufacturer. Even partial duplication of drawings,
images or text is prohibited.
The information contained in this document is subject to change
without prior notice.
Use restrictions
INTENDED USE
Lara sterilizers are widely used for medical purposes, e.g. in general
medical practices, dentistry, facilities for personal hygiene and
beauty care and also veterinary practices. They are also used for
materials and equipment that is likely to be exposed to blood or
body fluids, e.g. implements used by beauty therapists, tattooists,
body piercers and hairdressers.
The types of loads that can be sterilized with Lara sterilizers are
described in Table 1 of the reference technical norm EN 13060.
These loads include solid, porous, hollow loads type A and hollow
loads type B, unwrapped, single wrapped and double wrapped.
Lara sterilizers cannot be used to sterilize liquids or pharmaceutical
products.
The device is intended for professional use only by trained people.
PROVIDED FEATURES
See "Sterilization cycles" on page97 for the full list of key program
features, including sterilization time, temperature and recommended
load type.
USER QUALIFICATION
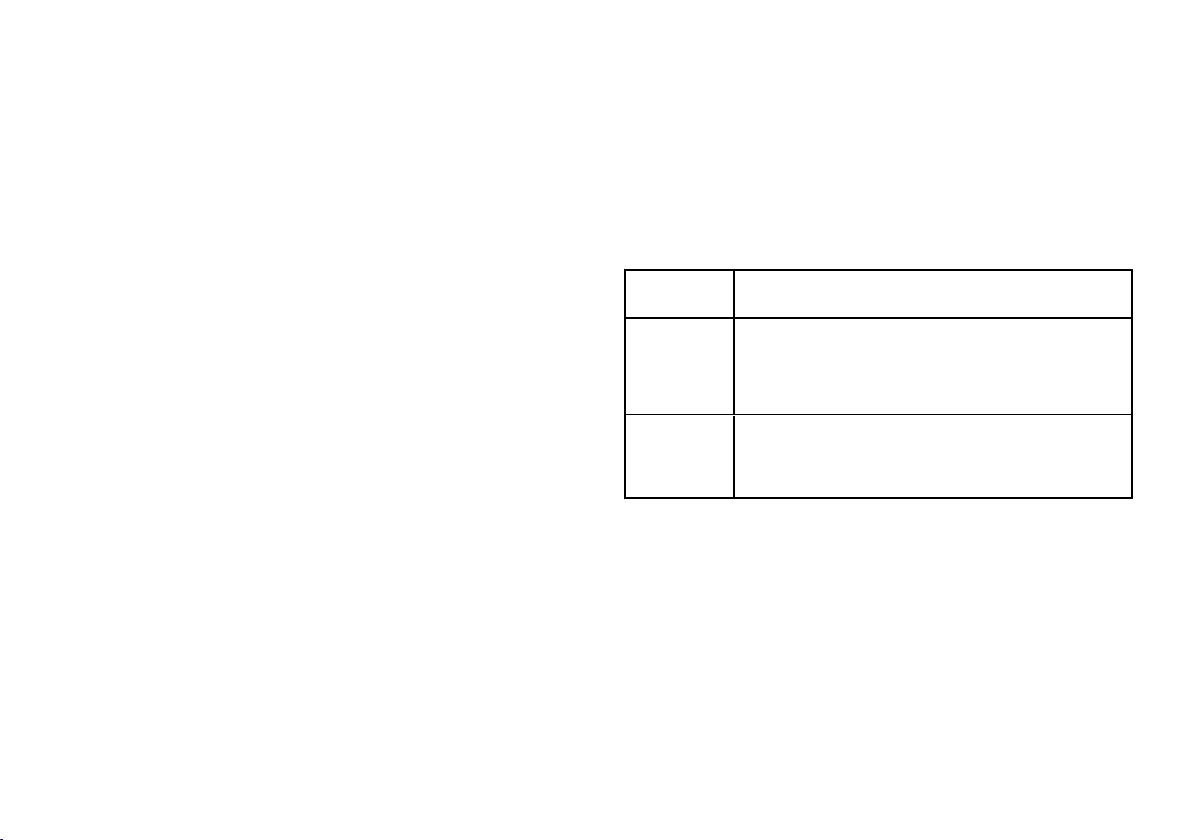
The users who may operate the sterilizer are the following.
User
qualification Competences
Head of the
clinic/practice
Legally responsible for:
nthe efficiency of the hygiene protocol in place
nthe sterilization process
nthe operators' training and training documentation
nthe correct operation and maintenance of the equipment
Trained
operators
nRegularly attend the training for operating and using the
sterilizer safely.
nUse the sterilizer according to the Head of the
clinic/practice's instructions.