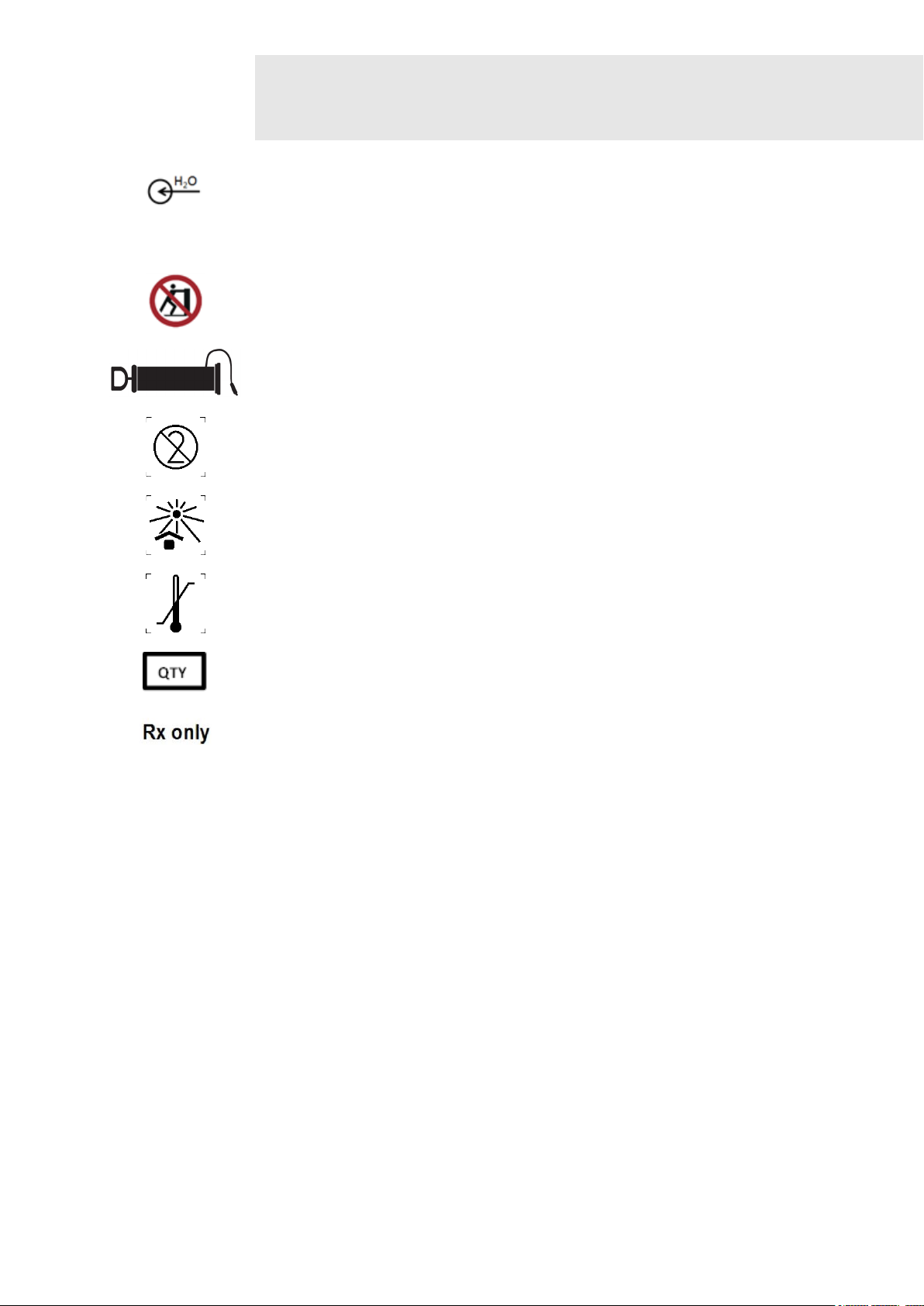
Connection for filling the cooling unit with water (max.1.5L)
The device must not be tilted.
Pump symbol indicating the location of the pump for water evacuation.
Do not reuse. If used more than once, cross infection may occur.
The transport package shall not be exposed to sunlight.
Symbol to indicate the maximum and minimum temperature limits at which the
item shall be stored, transported and used.
Quantity
Federal law restricts this device to sale by or on the order of a (licensed
healthcare practitioner