ActivePure F170A User manual

1
READ MANUAL CAREFULLY FOR PROPER USE AND OPTIMAL OPERATION.
MODEL F170A
The Aberdeen Bldg.
14841 Dallas Parkway, Suite 500
Dallas, TX 75254

2
IMPORTANT SAFETY & USE INSTRUCTIONS FOR THE ACTIVEPURE®
MEDICAL GUARDIAN
Read all instructions before using this device.
• Use only as described in this manual
• Do not attempt to repair or adjust any electrical or mechanical functions of the ActivePure® Medical Guardian. Contact Customer Service for
service support at 1.800.572.0317
• This device will automatically turn off when the door is open
• Do not use filters other than authorized ActivePure® Medical Guardian filters
• Do not place or operate device outdoors or on wet surfaces
• Do not look directly at the UV light inside unit when operating; eye damage may occur
• Do not allow device to be used as a toy; do not permit children to operate device
• Do not use with damaged cord or plug; if device is not working as intended, has been dropped, damaged, left outdoors, or is wet, contact
Customer Service for support at 1.800.572.0317
• Never pull or carry by cord, use cord as a handle, close door on cord, or pull cord around sharp edges or corners; keep cord away from
heated surfaces
• Do not unplug by pulling on cord; to unplug, grasp the plug to remove, not the cord
• Never handle plug or device with wet hands
• Keep hair, loose clothing, fingers, and all parts of body away from openings and moving parts
• Always turn off before unplugging
• To avoid electric shock or fire hazards, plug directly into an appropriate electrical outlet (see voltage listed on unit) using cable provided
• To reduce the risk of electrical shock, this device has a grounded plug (with a third prong); this plug will fit in a grounded outlet only one
way; if it does not fit, do not change the plug in any way; do not use adapters; contact a qualified electrician to install the proper outlet
• This device should not be used near the presence of combustible gasses
• Place this device in a location that allows air to move freely into, around and out of the device
When using an electrical device, basic precautions should always be taken.
WARNING:
SAVE THESE INSTRUCTIONS
This device should be maintained in accordance with the device Manual to prevent damage or risk of re.
CAUTION:
To reduce risk of re, electrical shock, or injury to persons, observe the following:
WARNING:
3
First, read this user manual in its entirety to ensure that the ActivePure® Medical Guardian device is used as intended. Then plug it in, turn it on to the appropriate
fan setting. The ActivePure® Medical Guardian is designed for quiet operation, as not to disturb people in a professional healthcare environment.
BEFORE YOU BEGIN USING THE ACTIVEPURE® MEDICAL GUARDIAN
INTENDED USE
FILL IN AND SAVE
Locate the serial number of your new ActivePure® Medical Guardian and write it here.
Retain it for future reference.
Model No. __________________________________________________________________
Serial No. __________________________________________________________________
Date of Purchase ____________________________________________________________
Distributor _________________________________________________________________
Distributor’s Oce Phone No.__________________________________________________
Distributor’s Oce Address ___________________________________________________
CONTENTS
Important Safety & Use Instructions . . . . . . . . . . . . 2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . 3
Device Description . . . . . . . . . . . . . . . . . . . . 4
Product Features . . . . . . . . . . . . . . . . . . . . . 5
Device Specications . . . . . . . . . . . . . . . . . . . 6
Operation. . . . . . . . . . . . . . . . . . . . . . . . . 6
Treatment Space . . . . . . . . . . . . . . . . . . . . . 7
Settings. . . . . . . . . . . . . . . . . . . . . . . . . . 8
General Maintenance Instructions . . . . . . . . . . . . . 8
Cleaning/Disinfecting and Filter Replacement Instructions . 9
ActivePure® Cell Replacement Instructions. . . . . . .11 - 12
Replacement Parts . . . . . . . . . . . . . . . . . . . .12
FAQs . . . . . . . . . . . . . . . . . . . . . . . . . . .12
Warranty Information . . . . . . . . . . . . . . . . . . .13
Device IEC 60601-1-2:2014 EMC Information . . . . . . . .14
The ActivePure® Medical Guardian, model F170A is a device intended for medical purposes that is used for the reduction of staphylococcus epidermidis and
erwinia herbicola bacteria, MS2 and Phi-X174 viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores from the air in a temperature-
controlled professional healthcare environment of 70~71°F, 40~45% RH.
The ActivePure® Medical Guardian, model F170A has demonstrated the reduction of staphylococcus epidermidis and erwinia herbicola, bacteria MS2 and Phi-X174
viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores under the following conditions;
Organism Type Organism Name Test Temp/RH Exposure Time (m) Avg Log-Reduction
Bacteria Staphylococcus epidermidis 72°F/50% 60 5.95
Bacteria Erwinia herbicola 72°F/50% 60 5.12
Virus MS2 bacteriophage 72°F/50% 60 5.58
Virus Phi-X174 72°F/50% 60 4.19
Fungal spore Aspergillus niger 72°F/50% 60 4.12
Bacterial spore Bacillus globigii 72°F/50% 60 4.22
4
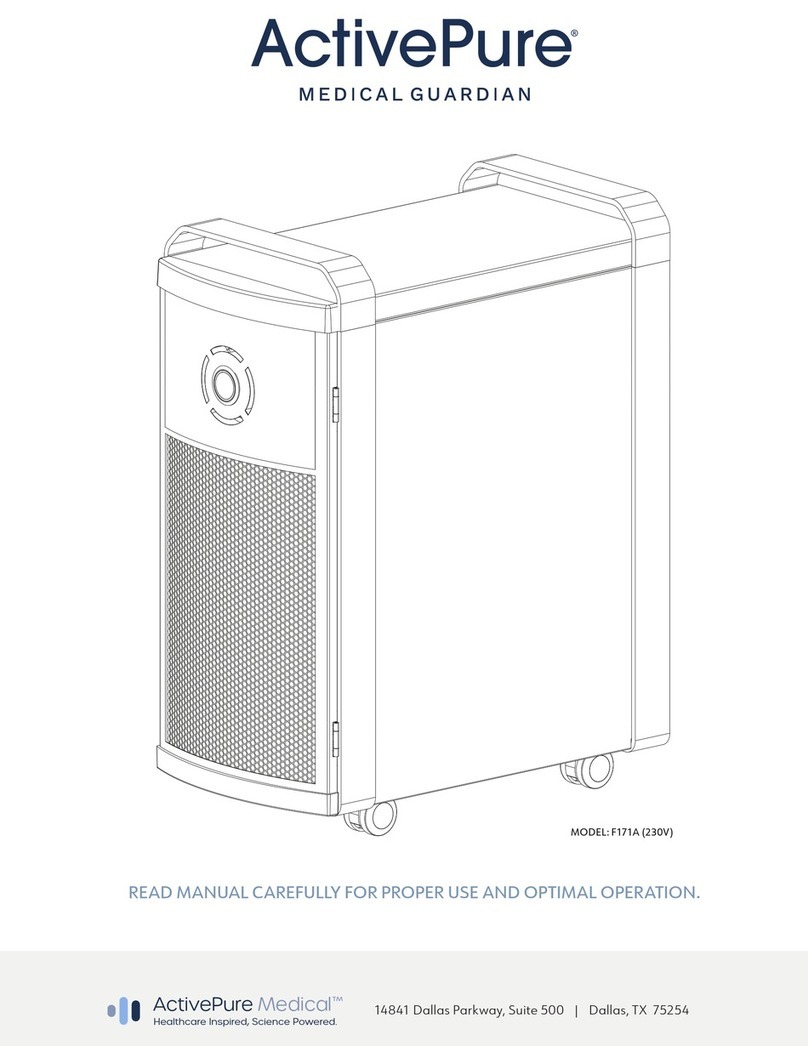
DEVICE DESCRIPTION
The ActivePure® Medical Guardian, model Number F170A is a free-standing portable device, manufactured in the USA, constructed of aluminum
and steel, dimensionally measures 11.5” W x 26.5” H x 21.0” D and weighs 48 lbs. The device operates using a standard 120 VAC, 60Hz power source.
Power consumption ranges from 1 watt in standby mode to 117 watts on high speed. The device includes: a HEPA lter; multipoint ionizer; a
photocatalyst module consisting of two 10-watt UVGI bulbs operating at a wavelength of 254nm; two pieces of polycarbonate honeycomb material
with a combined surface area of 708 square inches, coated with a TiO2 based catalyst called ActivePure®; power switch; circuit board which controls
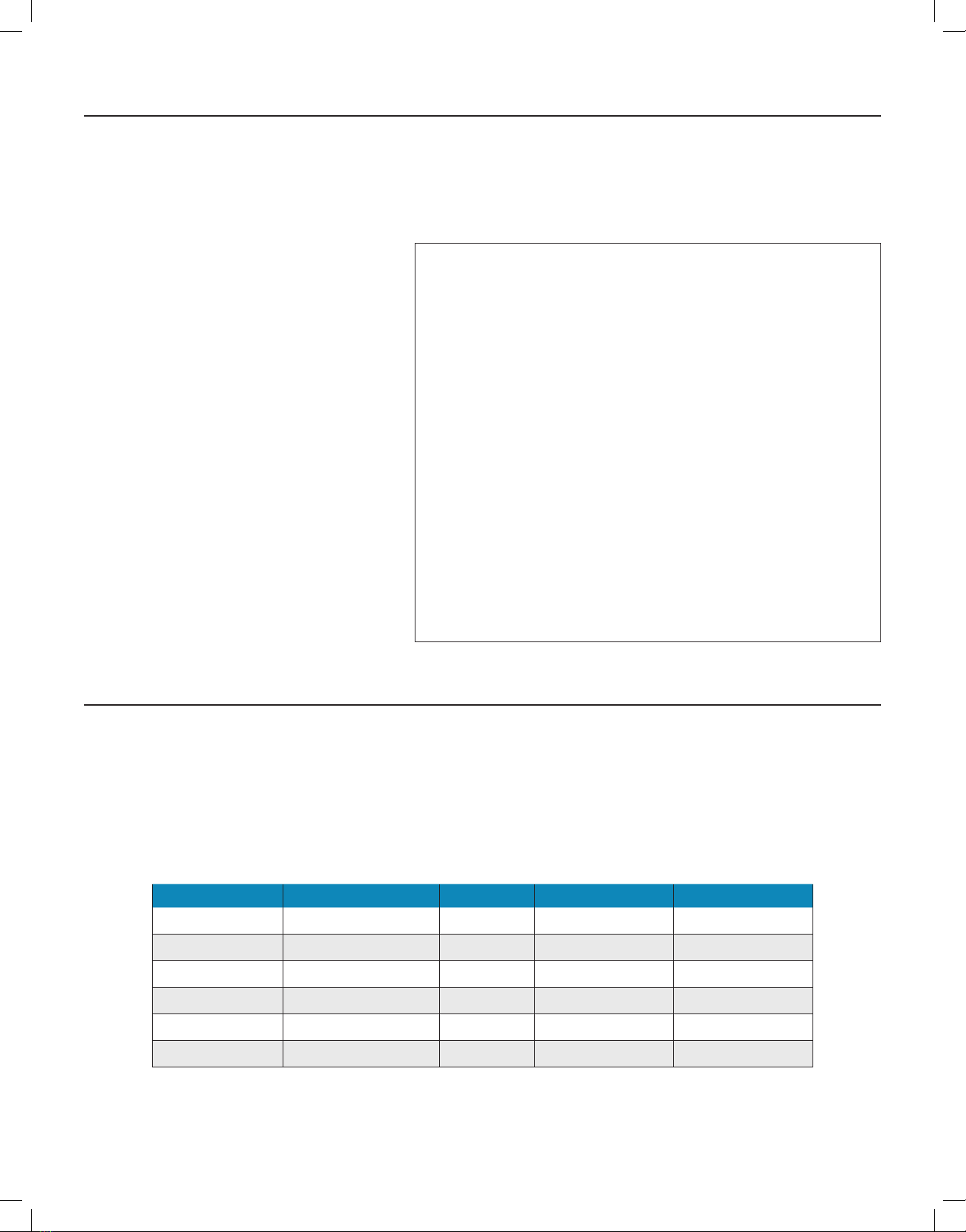
the device operations and an internal fan to draw air through the device, as shown in the general device design (Figure 1).
Lab test results demonstrated the ActivePure® Medical Guardian, model F170A device using ActivePure® to expose Staphylococcus epidermis and MS2
bacteriophage to hydroxyl radicals and super ions formed as the result of UV exposure on the photo catalyst, has the ability to destroy the tested bio
organisms on the lter as shown in Table 1 below.
The bio organisms selected, Staphylococcus epidermidis, a bacteria and MS2 bacteriophage, a virus would provide a strong challenge against the
ActivePure® hydroxyl radicals and super ions exposure.
"ActivePure®"
Figure 1
Organism Type Organism Name Test Temp / RH Exposure Time (H) Avg Log – Reduction
Bacteria, gram + Staphylococcus epidermidis 73.2°F / 50% 1 5.77
Virus, RNA MS2 bacteriophage 73.2°F / 50% 72 4.15
Table 1

5
PRODUCT FEATURES
It is important to be familiar with the components of the ActivePure® Medical Guardian. If any components are missing, contact Customer Service
HEPA Filter Chamber
(Filter not shown)
Airflow
Input
Door Pull
Handle
ActivePure® Cell
Power Cord
(Plug not
shown)
UNIQUE FEATURES
• Flow-through air design provides less air resistance
• Multi-point ionization creates negatively charged particles which ow out from a series of carbon brushes
• Carbon to remove odors
• Hydrophobic polypropylene pleated media lter designed to reduce airborne contaminants
• Powerful UVGI bulbs
• Photo catalyst module using ActivePure®
• 4-Speed fan draws airborne contaminants through the device
• All steel and aluminum construction for durability and long life
• Automatic ambient light sensor automatically dims display in darkened environment
• Safety shutdown which prevents continued device use once lter and/or ActivePure® Cell replacement time is reached
• Made in the USA
Airflow
Output
On/Off
Speed Control
Front
Panel
Back
Panel

6
MODEL: F170A
Operation Voltage: 120 VAV, 60 Hz
Power Button Function: ON / 1 / 2 / 3 / 4 / OFF
Operational Indicators:
Green ring – power on, device in operation
Filter change – activates after 180 days of usage
Photocatalyst cell (ActivePure®) – activates after 365 days of usage
Reset Button:
Press and hold power button for 10 seconds to reset lter and/or cell
indicators
Ionizer Voltage: 4.5 ~ 5.5 kV
Negative Ion Output (Avg): 8.73 x 106/ cm3
Treatment Area: 3,000 ft3with ceilings 8 ~ 10 ft
Recommended Fan Speed: High
Fan Speeds / Fan Indicators: 4
Whisper | 1 blue bar is illuminated
Low | 2 blue bars are illuminated
Medium | 3 blue bars are illuminated
High | 4 blue bars are illuminated
Nominal Airow Rate:
Whisper | 90 CFM | 53 m3/h
Low | 100 CFM | 59 m3/h
Medium | 180 CFM | 305 m3/h
High | 300 CFM | 509 m3/h
OPERATION
DEVICE SPECIFICATIONS
Place the ActivePure® Medical Guardian in a location that allows air to move freely into, around and out of the unit. Allow minimum of 12" of
clearance around intake grill (airow input).
DO NOT place the unit:
• Directly on or near soft furnishing (such as bedding or curtains)
• Near sources of heat (such ad radiators, replaces or ovens)
• Near combustible gasses
• Near water, outdoors or in a damp or wet area
WARNING:
Nominal Wattage:
Standby 1
Whisper 41
Low 46
Medium 60
High 117
Average Sound (dbA)(i):
Whisper 43
Low 48
Medium 64
High 70
(measured @1m from front)
Dimensions:
26.5"H x 11.5"W x 21.0"D
673mm H x 292mm W x 533mm D
Weight:
48 lbs.
21.8 kg
Power Cord: 6 ft.
ActivePure® Medical, LLC reserves the right to change or modify any
specications without notice. Consult www.ActivePureMedical.com for the
latest device information or consult Customer Service at 1.800.572.0317.
Life:
3-years minimum operational life
7
TREATMENT SPACE
The ActivePure® Medical Guardian, model F170A is a device intended for medical purposes that is used in professional healthcare environments. The device
is designed to treat a space approximately 3,000 cubic feet that would have 8’ to 10’ ceilings. Recommended mode of action is to operate the device on the
highest speed possible continuously. The device recirculates ambient air continuously through the device where the lter using straining and attraction reduces
airborne contaminants from the air. Hydroxyl radicals and super ions created by the photo catalyst ActivePure® are mixed with the contaminant reduced air
which then enters the treatment space. The ActivePure® photo catalyst reduces the viability of microorganisms 4-6 log such as Staphylococcus epidermidis and
MS2 as tested, shown in Table 3. Larger spaces or operating on lower fan speeds may require multiple devices. Operational guidelines are provided to users in
the form of an Owner’s Manual. The length of time required to reach optimal reduction varies as a function of space volume and the dynamics of what occurs
within the treatment space including:
• Degree of contaminants in the air or being introduce by activities in the space
• Movement of people or equipment within the space
• Rates of ventilation
The device is intended to result in a 4-6 log reduction of airborne microorganisms from initial concentrations in 60 minutes as shown in Table 1. The ActivePure®
Medical Guardian, model F170A is not a sterile device and is not intended to create a sterile environment.
Organism
Type Organism Name Initial Load of
Microorganisms
Avg Log-Reduction after
60 minutes
Bacteria (gram +) Staphylococcus epidermidis 2.6e5cfu/L 5.95
Bacteria (gram -) Erwinia herbicola 1.9e5cfu/L 5.12
Virus (RNA) MS2 8.1e5cfu/L 5.58
Virus (DNA) Phi-X174 7.63e3cfu/L 4.19
Fungal spore Aspergillus niger 2.61e4cfu/L 4.12
Virus (DNA) Baillus globigii 7.95e5cfu/L 4.22
Fan Speed CFM of Air Movement Cubic Feet Air
Movement in 1 Hour
Air Exchanges per Hour
based on 3,000 ft3
Whisper 90 5,400 1.8
Low 100 6,000 2.0
Medium 180 10,800 3.6
High 300 18,000 6.0
Table 1
Table 2
Table 3
The information on the device CFM performance and air exchanges for the dierent speed settings is shown in Table 2, to illustrate the inuence of treatment
space to air exchanges on dierent fan speeds. These are provided to assist in determining the number of devices or fan speed settings necessary for a given space.
Organism Type Organism Name Test Temp / RH Exposure Time (H) Avg Log – Reduction
Bacteria, gram + Staphylococcus epidermidis 73.2°F / 50% 1 5.77
Virus, RNA MS2 bacteriophage 73.2°F / 50% 72 4.15

8
The ActivePure® Medical Guardian is designed to reduce airborne contaminants with minimal maintenance.
Filter: The ActivePure® Medical Guardian HEPA Filter is replaced when the change lter light illuminates after 180 days
(approx 6 months) of use.
Cell Module: The ActivePure® Medical Guardian ActivePure® Cell is replaced when the change cell light illuminates after
365 days (1 year) of use.
The ActivePure® Medical Guardian has a safety shutdown feature to ensure the lter and/or cell replacement is performed
at the appropriate times. When the lter and/or cell replacement time is reached, the appropriate LED indicator will
illuminate. However, if the replacement indicator(s) are ignored or if the one or both LED's fail to illuminate, by design
the device will shut down 3 days of operation after reaching the replacement time. This is to ensure the device does not
continue to operate with a lter and/or cell past its stated life.
Cabinet: Wipe the exterior of the cabinet with a clean, soft cloth as needed to remove nger prints, smudges, etc. Clean
and disinfect device after each lter change as detailed in the Cleaning/Disinfecting and Filter Replacement
Instructions.
Important Points:
• Always unplug device before cleaning
• Never use gasoline, chemical solvents or any type of corrosive material to prevent damage to the device
• Use genuine ActivePure Medical® parts to maintain the warranty (See Warranty on Page 11)
SETTINGS
NOTE: Safety switch automatically turns power o when door is opened.
There are four fan settings on the ActivePure® Medical Guardian:
1. Whisper (one blue light illuminated)
2. Low (for normal use... two blue lights illuminated)
3. Medium (for a larger areas... three blue lights illuminated)
4. High (when maximum airow is desired... four blue lights illuminated)
When operating the ActivePure® Medical Guardian device for the rst time, set the fan speed to the
highest appropriate speed for the area its placed. Then adjust fan as appropriate for the area if needed.
GENERAL MAINTENANCE INSTRUCTIONS
On/Off
Speed Control
(green light illuminates
when power is on)
Disconnect power before servicing.
CAUTION:

9
CLEANING/DISINFECTING AND FILTER REPLACEMENT INSTRUCTIONS
The ActivePure® Medical Guardian, model F170A is a device intended for medical purposes that is used for the reduction of staphylococcus
epidermidis and erwinia herbicola bacteria, MS2 and Phi-X174 viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores from
the air in a temperature-controlled professional healthcare environment of 70~71°F, 40~45%RH.
The following instructions cover device cleaning, disinfecting and replacement of the lter. Thoroughly read all of the instructions before starting. It
is recommended to clean and disinfect the device each time the lter is replaced or when device becomes visibly soiled.
FILTER REPLACEMENT
Metrex CaviWipes are required to clean the device when replacing the lter, but they are not provided with the unit or lter.
Step 1: Always disconnect (unplug) device from electrical power source before cleaning, disinfecting or replacing the lter.
Step 2: If necessary, relocate the device to an appropriate area to perform the cleaning, disinfecting and lter replacement. It is not recommended
to perform these tasks in areas such as patient room, operating room, examination room, etc., where patient exposure may occur.
Step 3: Open the replacement lter carton which includes the following items inside.
• 1 replacement lter in a sealed wrapper
• 1 disposable surgical mask
• 1 pair disposable latex free gloves, large
• 1 disposable 8-quadrant wiping cloth
• 1 large plastic bag
• 1 red bio hazard sticker
• 1 plastic bag seal
• 1 printed copy of instruction for use, cleaning, disinfecting, lter replacement sheet
Step 4: Before removing and handling the used lter, put on the disposable mask and gloves.
Step 5: Slide (remove) the used lter from the device, placing it inside the large plastic bag provided for disposal,
then set it aside.
Step 6: Use Metrex CaviWipes to clean the device. These are NOT provided with the device or replacement lter.
NOTE: Metrex CaviWipes are required to clean the device when replacing the lter, but they are not provided with the
unit or lter.
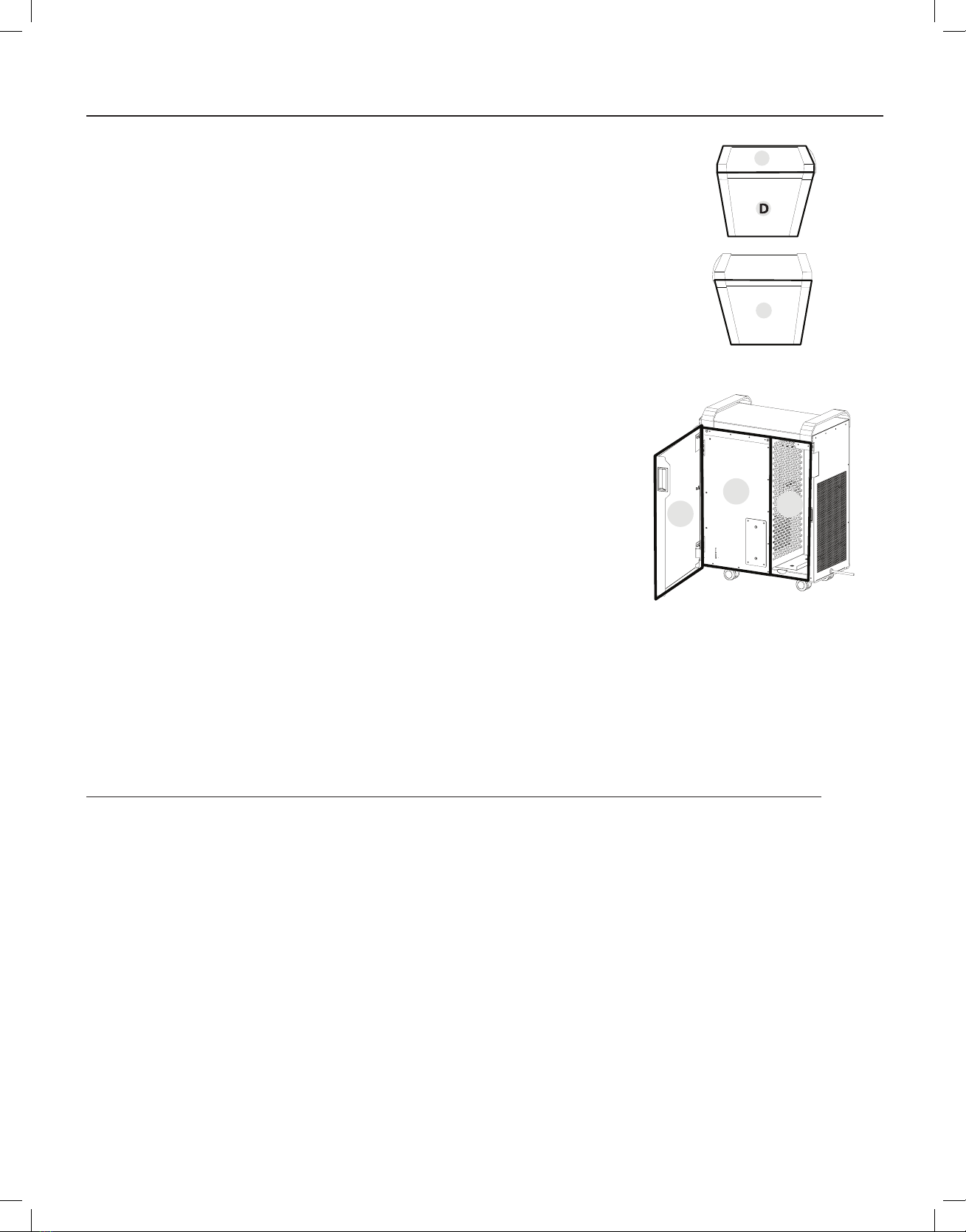
Step 7: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “A”. Wipe the CaviWipe over the surface area,
thoroughly wetting the soil. Then using hand pressure rub the wipe over the surface until soil is no longer visible on this part of
the device. If soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
Step 8: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “B”. Wipe the CaviWipe over the surface area,
thoroughly wetting the soil. Then using hand pressure rub the wipe over the surface until soil is no longer visible on this part of
the device. If soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
Step 9: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “C”. Wipe the CaviWipe over the surface area,
thoroughly wetting the soil. Then using hand pressure rub the wipe over the surface until soil is no longer visible on this part of
the device. If soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
1
2
3
4
5
A
B
C
10
Step 10: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “D”. Wipe the CaviWipe over
the surface area, thoroughly wetting the soil. Wait 1 minute, then using hand pressure rub the wipe over the
surface until soil is no longer visible on this part of the device. If soil is heavy or is still visible, repeat step with a
new CaviWipe until soil is no longer visible.
Step 11: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “E”. Wipe the CaviWipe over
the surface area, thoroughly wetting the soil. Wait 1 minute, then using hand pressure rub the wipe over
the surface until soil is no longer visible on this part of the device. If soil is heavy or is still visible, repeat step
with a new CaviWipe until soil is no longer visible.
Step 12: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “F”. Wipe the
CaviWipe over the surface area, thoroughly wetting the soil. Wait 1 minute, then using hand
pressure rub the wipe over the surface until soil is no longer visible on this part of the device. If
soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
Step 13: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “G”. Wipe the
CaviWipe over the surface area, thoroughly wetting the soil. Wait 1 minute, then using hand
pressure rub the wipe over the surface until soil is no longer visible on this part of the device. If
soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
Step 14: Remove a Metrex CaviWipe from its package. Beginning by cleaning the surface “H”. Wipe the
CaviWipe over the surface area, thoroughly wetting the soil. Wait 1 minute, then using hand
pressure rub the wipe over the surface until soil is no longer visible on this part of the device. If
soil is heavy or is still visible, repeat step with a new CaviWipe until soil is no longer visible.
Step 15: Allow the device to dry, no visible wetting remains. No rinsing is required. With the device
cleaned, it is ready for disinfecting.
DISINFECTING
Disinfectants are typically provided as a concentrate to be mixed with water or ready to use (RTU) with no mixing required. Use Clorox
Healthcare Fuzion Cleaner Disinfectant RTU in a spray bottle. As an RTU product, no mixing is necessary. Clorox recommended disinfectant
wait time is 1 minute with no rinsing required.
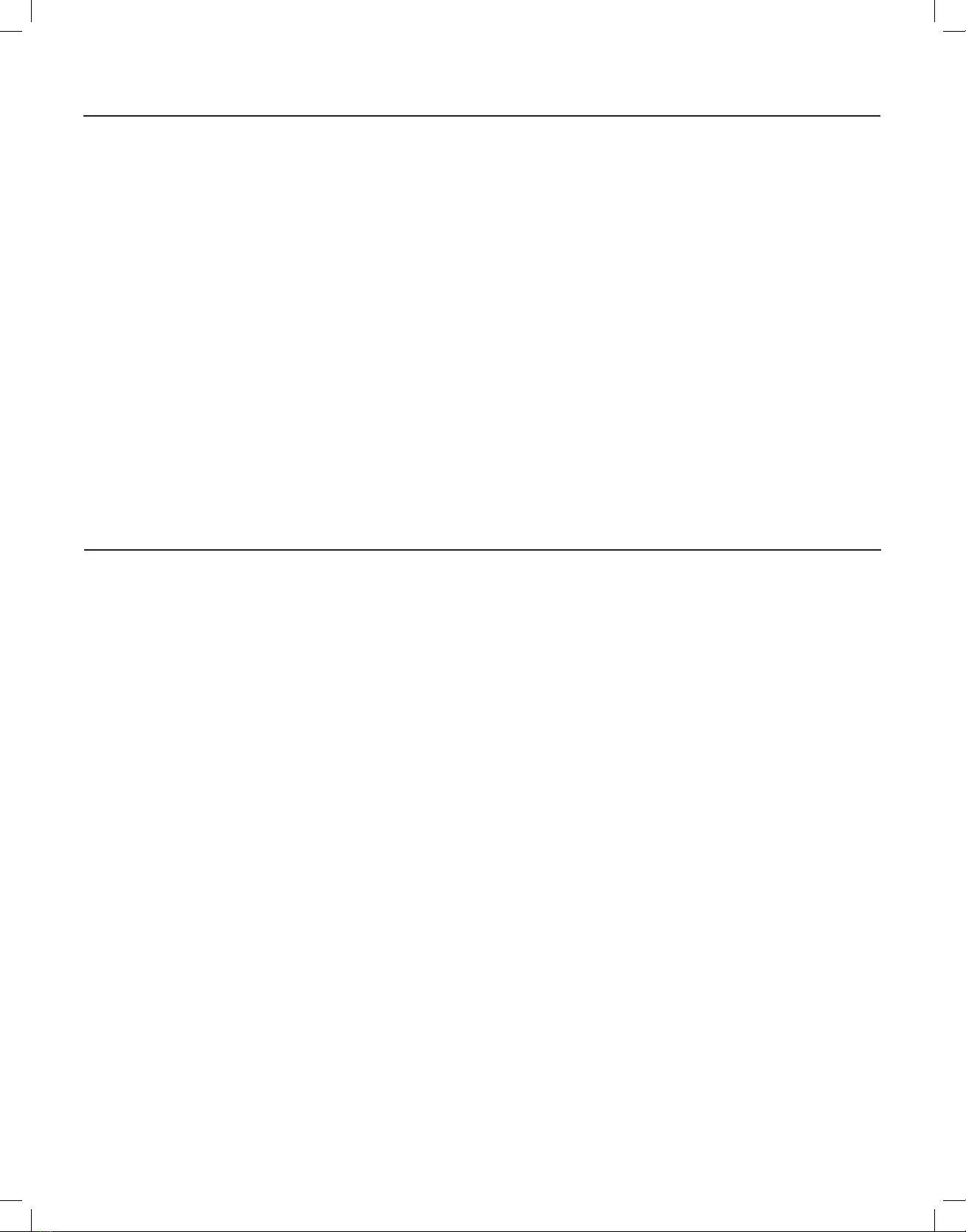
In Steps 16 - 24, spray Clorox Healthcare Fuzion Cleaner Disinfectant liberally on each surface until that surface is thoroughly wetted.
Step 16: Take the 8-quadrant wiping cloth from the lter carton, folding it as before so quadrant 1 is showing.
Step 17: Spray disinfecting solution over surface A. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 1. Continue wiping
until all solution has been removed.
Step 18: Spray disinfecting solution over surface B. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 2. Continue wiping
until all solution has been removed.
Step 19: Spray disinfecting solution over surface C. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 3. Continue wiping
until all solution has been removed.
Step 20: Spray disinfecting solution over surface D. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 4. Continue wiping
until all solution has been removed
Step 21: Spray disinfecting solution over surface E. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 5. Continue wiping
until all solution has been removed.
Step 22: Spray disinfecting solution over surface F. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 6. Continue wiping
until all solution has been removed.
CLEANING/DISINFECTING AND FILTER REPLACEMENT INSTRUCTIONS CONT.
E
FGH
C
11
CLEANING/DISINFECTING AND FILTER REPLACEMENT INSTRUCTIONS CONT.
Step 23: Spray disinfecting solution over surface G. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 7. Continue wiping
until all solution has been removed.
Step 24: Spray disinfecting solution over surface H. Wait 4 minutes, ± 5 seconds, then thoroughly wipe with the cloth folded to quadrant 8. Continue wiping
until all solution has been removed.
Step 25: When nished, place the disposable mask, gloves and wiping cloth in the large plastic bag with the used lter.
Step 26: Secure the large plastic bag with the provided bag closure and place the red biohazard label on the plastic bag. Disposal to be in accordance with
facility policy.
Step 27: Remove the new lter from its protective wrap and slide (following directions on lter) it into the device. The protective lter wrap is not a biohazard,
so no special handling is required.
Step 28: Allow the device to dry, no visible wetting remains, and no rinsing is required. Plug device in and perform a reset of the lter change light by pressing
and holding the power button for 10 seconds until the lter replace light goes out.
Step 29: The device is now ready to be returned to use.
ACTIVEPURE® CELL REPLACEMENT INSTRUCTIONS
The ActivePure® Medical Guardian, model F170A is a device intended for medical purposes that is used for the reduction of staphylococcus epidermidis and
erwinia herbicola bacteria, MS2 and Phi-X174 viruses and aspergillum niger fungal spores and bacillus globigii bacterial spores from the air, in a temperature-
controlled professional healthcare environment of 70~71°F, 40~45% RH.
The following instructions will permit the device ActivePure Cell to be safely replaced without creating safety concerns in the patient
environment.
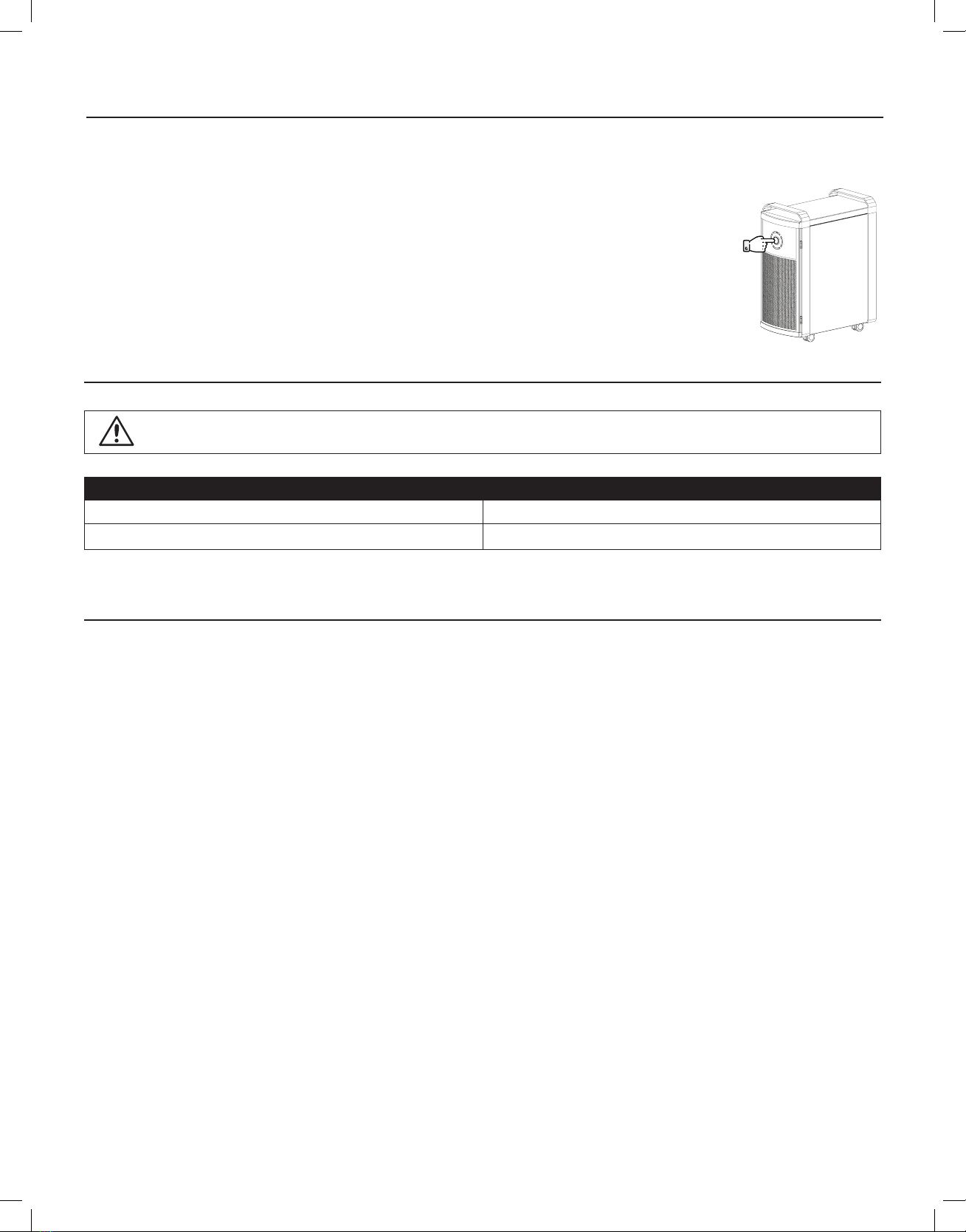
Step 1: Always disconnect (unplug) device from electrical power source before replacing the ActivePure Cell.
Step 2: If necessary, relocate the device to an appropriate area to perform cell replacement. Do not replace cell in areas such as patient room, operating room,
examination room, etc., where patient exposure may occur.
Step 3: Remove the 4 screws securing the old cell mounting plate on the device.
Step 4: Slide the old cell out of the device and disconnect the two bulb sockets.
Step 5: Open the replacement cell carton and remove the new cell.
Step 6: Place old cell inside the empty new cell carton and dispose of properly.
NOTE: No special handling for contaminants is necessary as the cell is on the exhaust side of the device and does not trap or contain contaminants.
Step 7: Reattach the two bulb sockets to the new cell.
Step 8: Slide the new cell back inside the device and secure with the 4 screws removed in Step 3.
Step 9: Plug device in and reset the ActivePure Cell Change Light by pressing and holding the power button for 10 seconds until it goes out.
Step 10: The device is now ready to be returned to its location.
12
ACTIVEPURE® CELL REPLACEMENT INSTRUCTIONS CONT.
FREQUENTLY ASKED QUESTIONS
REPLACEMENT PARTS
REPLACEMENT PARTS QTY Part Number
HEPA Filter (1) 49939
ActivePure® Cell 49940
After you replace the filter or ActivePure Cell you will want to reset the indicator light(s). To do so simply press
and hold the power button for 10 seconds. This will reset the filter and/or cell module replacement indicators.
Only the illuminated indicator(s) will be reset when pressing the power button for 10 seconds.
Q: When the device is first turned on, the fan runs at a higher speed for a second or two before running on the low setting?
A: The fan on whisper runs very slow so the device provides extra power to the fan for a second or two on start up to ensure the fan is
operating.
Q: When I select different fan speeds, why do I hear a clicking sound?
A: The clicking sound is a mechanical relay closing for that speed setting.
Q: When I turn off the device, why does the fan continue to run for a short time?
A: This 3 second delay provides longer fan life.
Q: Why doesn't the display dim immediately when the room gets dark?
A: The automatic dim feature has a brief delay to prevent unnecessary dimming if a shadow momentarily passes in front.
Q: How will I know to change the filter or ActivePure® Cell?
A: The device control panel will illuminate a change filter or cell icon when its time to change one or both.
Q: What if I ignore the illuminated icons and decide not to change the filter or cell?
A: The device has a safety feature to prevent the continued use of the device 3 days after it is time to change the filter or cell.
Q: How do I reprocess the device?
A: Follow the instruction inside the user manual, on the website or what comes with a replacement filter. These instruction details the
cleaning and disinfecting procedure.
Q: What if I have question about using the device?
RESETTING FILTER AND/OR ACTIVEPURE PHOTOCATALYST CELL MODULE INDICATORS Press and hold for
10 seconds
Use only ActivePure® Medical, LLC recommended Replacement Parts.
WARNING:
If you have questions regarding replacement parts and service, contact Customer Service at 1.800.572.0317.

13
LIMITED ONE 1 YEAR WARRANTY
WHAT IS COVERED BY THIS WARRANTY
ActivePure® Medical, LLC warrants the ActivePure® Medical Guardian, to
the original purchaser subject to the conditions below, against defects in
workmanship or material, provided that the products are returned to an
authorized location within 1 year of date of purchase*.
MAINTENANCE REQUIREMENTS
Failure to use and maintain the ActivePure® Medical Guardian in accordance
with the Owner’s Manual will void this warranty, including but not limited to,
replacing your HEPA lters with genuine lters every 180 days (6 months) of
use, and replacing the ActivePure® Cell with a genuine ActivePure® Cell every
365 days (1 year) of use. Proof of change may be required. Servicing your
ActivePure® Medical Guardian by parties other than an authorized location
and/or using parts other than genuine ActivePure® Medical parts will also
void this warranty.
HOW TO OBTAIN WARRANTY SERVICE
Customers must contact their distributor or Customer Service and provide
proof of purchase within the above time periods. We will repair or replace
and return the device, without charge and within a reasonable period of
time, subject to the conditions herein, if our examination shall disclose any
part to be defective in workmanship or material. If we, in our discretion, are
unable to repair the device after a reasonable number of attempts, we will
provide either a refund of the purchase price or a replacement device, at the
company’s option.
WHAT IS NOT COVERED BY THIS WARRANTY
This device is intended for use in a professional healthcare environment
only. Ordinary wear and tear shall not be considered a defect in
workmanship or material. These warranties do not apply for loss or damage
caused by accident, re, abuse, misuse, modication, misapplication, or by
any repairs other than those provided by an authorized location.
EXCLUSION OF OTHER WARRANTIES AND CONDITIONS
EXCEPT AS PROVIDED HEREIN, ACTIVEPURE® MEDICAL, LLC MAKES NO
REPRESENTATION OR WARRANTY OF ANY KIND. ALL OTHER WARRANTIES
OF ANY KIND, EXPRESS OR IMPLIED, ARE HEREBY EXPRESSLY DISCLAIMED,
INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS
FOR A PARTICULAR PURPOSE.
UNAUTHORIZED CHANNELS & MISSING SERIAL NUMBERS
If a valid serial number is missing from the device, the warranty will be
voided. Genuine devices are permitted to be sold through our authorized
representatives only. Warranties are voided if a device is purchased through
unauthorized channels, this includes websites that are not authorized to
use ActivePure® Medical's trademarked names, images and logos as well as
Internet auction sites (e.g. ebay and Craigslist). The only approved Internet
presence for ActivePure® Medical, LLC products is www.activepuremedical.
com. To conrm warranty coverage prior to purchasing a device, contact
Customer Service at 1.800.572.0317 with the serial number.
LIMITATION OF LIABILITY FOR SPECIAL, INCIDENTAL OR
CONSEQUENTIAL DAMAGES
ACTIVEPURE® MEDICAL, LLC SHALL NOT IN ANY CASE BE LIABLE FOR
SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES ARISING FROM
BREACH OF EXPRESS OR IMPLIED WARRANTIES, CONDITIONS, GUARANTEES
OR REPRESENTATIONS, BREACH OF CONTRACT, NEGLIGENCE OR ANY OTHER
LEGAL THEORY. Such excluded damages include, but are not limited to, loss
of prots or revenue and loss of the use of the products.
FOR U.S. APPLICATION ONLY
This warranty gives you specic legal rights, and you may also have other
rights which vary from state to state. Some states do not allow limitations
on warranties, or on remedies for breach. In such states, the above
limitations may not apply to you.
FOR CANADIAN APPLICATION ONLY
Exclusion of Subsequent Owners: Except as otherwise required by
applicable legislation, this warranty is not transferable. This warranty gives
you specic legal rights and you may also have other rights which vary
from province to province. Some provinces and territories do not allow
limitations on warranties, or on remedies for breach. In such provinces or
territories, the above limitations may not apply to you.
PREVENTATIVE MAINTENANCE PROGRAM
Preventative maintenance programs may be available. These programs
help maintain the device’s optimal performance and may extend the life. To
nd out if a preventative maintenance program is available for this device,
please contact an authorized representative or Customer Care. Terms and
conditions will be all as set forth in the preventative maintenance program
documents.
If any provision of this warranty or part thereof is held by a court of
competent jurisdiction to be invalid, illegal or unenforceable, the validity,
legality and enforceability of the remaining provisions or parts thereof will
not in any way be aected or impaired within the jurisdiction of that court.
This entire warranty shall continue to be valid, legal and enforceable in any
jurisdiction where a similar determination has not been made.
This warranty is provided by:
ActivePure Medical, LLC
The Aberdeen Bldg.
14841 Dallas Parkway, Suite 500
Dallas, TX 75254
SERVICE
From time to time, modications to our devices may occur without notice.
For the latest information, please call customer service at 1.800.572.0317 or
email at medical@activepure.com.
*Warranty does not include consumable items.

14
DEVICE IEC 6060112:2014 EMC INFORMATION
This device passed each of the 11 tests, as indicated. Immunity test levels performed on the device,
• Electrostatic Discharge Immunity – Tested at 2,4,8 and 15 kV
• Radiated Immunity – Tested 3 V/m (Professional Healthcare) and 10 V/m (Home Healthcare)
• Conducted Immunity – Tested 150 Hz ~ 80 MHz with AM 80% @ 1kHz 3V and 6V
TEST STANDARD
Conducted Emissions (EN 55011, CISPR 11)
Radiated Emissions (EN 55011, CISPR 11)
Harmonics (EN 61000-3-2)
Flicker (EN 61000-3-3: IEC 61000-4-2)
Electrostatic Discharge (EN 61000-4-2: IEC 6100-4-2)
Radiated Immunity (EN 61000-4-3: IEC 61000-4-3)
Fast Transient Burst (EN 61000-4-4: IEC 61000-4-4)
Surges (EN 61000-4-5: IEC 61000-4-5)
Conducted Immunity (EN 61000-4-6: IEC 61000-4-6)
Power Frequency Magnetic Field (EN 61000-4-8: IEC 61000-4-8)
Voltage Dips and Interrupt (EN 61000-4-11: IEC 61000-4-11)
• This device conforms fully to the EMC requirement of IEC 60601-1-2 and is suitable for use in a professional healthcare environment
• This device is not effected by RFID, wireless networks, 2-ways radios, paging systems, etc., as the device does not transmit or receive RF
signals or create EMF beyond the required limits
• Do not use device with an extension cord as unintended EMF may be created
• This device is grounded to prevent electrostatic discharge
• Do not use RF communication or magnetic field generating equipment within 30 cm of the device
• EMC testing shows no conducted or radiated electromagnetic emissions or immunity issues which would be adverse to the patient or
operator
The use of this device in a manner other than described in the user manual or a modication of the device could result in increased
electromagnetic emissions or decreased immunity.
If device is operating in an unexpected manner or causing unexpected interference , discontinue using the device, review the user
manual or seek service.

15

16
16
© ActivePure® Medical, LLC. All Rights Reserved
APMG_OM_65-00821_VA-01571_0321
www.activepuremedical.com
ActivePure® Medical, LLC | The Aberdeen Bldg. | 14841 Dallas Parkway, Suite 500 | Dallas, TX 75254 | 1.800.572.0317
ActivePure® Medical, LLC Canada, Inc. | 3480 Laird Road, Suite 2-5 | Mississauga, ON L5L 5Y4
For information regarding the use of this product please call Customer Service.
1.800.572.0317
or Email at
Table of contents
Other ActivePure Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual