NeuroBlate® System Instructions for Use
80469 Rev D – Nov 16, 2018
- 3 -
5. MRI CONDITIONAL STATUS......................................................................................................................... 34
5.1. MR IMAGE ARTIFACT ................................................................................................................................36
6. POTENTIAL HARMS OR HAZARDS ............................................................................................................... 38
7. NEUROBLATE® SYSTEM SETUP.................................................................................................................... 39
7.1. MRI PROTOCOLS AND DICOM IMAGE TRANSFER SETUP ......................................................................................39
7.2. CO2TANKS..................................................................................................................................................43
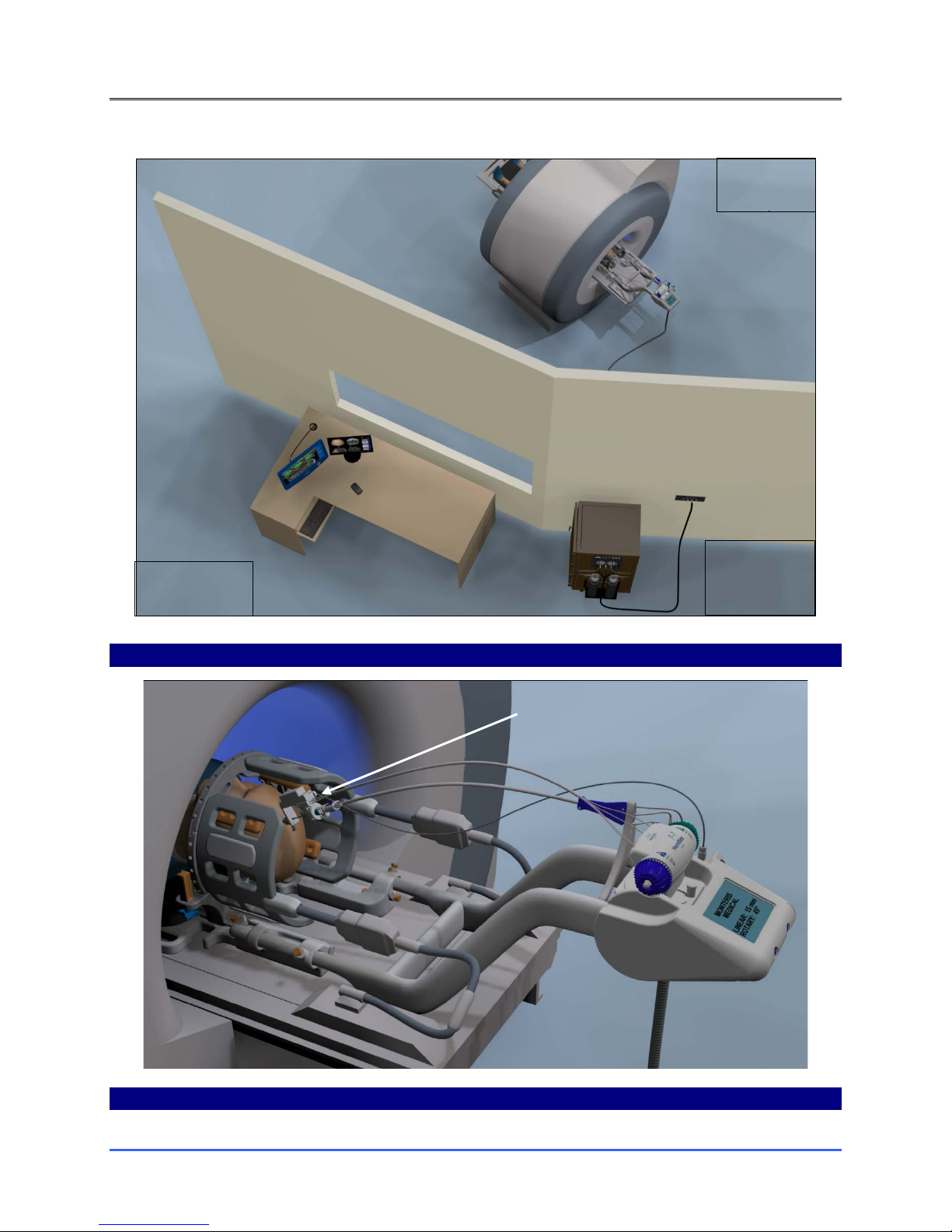
7.3. MRI SHIELD PENETRATION FOR NEUROBLATE CABLING ........................................................................................45
7.4. NEUROBLATE SYSTEM CONTROL WORKSTATION: ................................................................................................47
7.4.1. Software Boot Up and Launch Screen..................................................................................................48
7.5. NEUROBLATE SYSTEM M*VISION™SOFTWARE APPLICATION................................................................................49
7.5.1. Workflow Task Icons and Description..................................................................................................50
7.5.2. Main Menu Bar Options.......................................................................................................................51
7.5.3. Toolbar and System Status Description ...............................................................................................52
7.5.4. Hardware Interfaces ............................................................................................................................54
7.5.5. MR Thermal Data Transfer ..................................................................................................................54
7.5.6. Initiating Data Transfer Interface ........................................................................................................54
7.5.7. Thermal Monitoring Slice Planes .........................................................................................................55
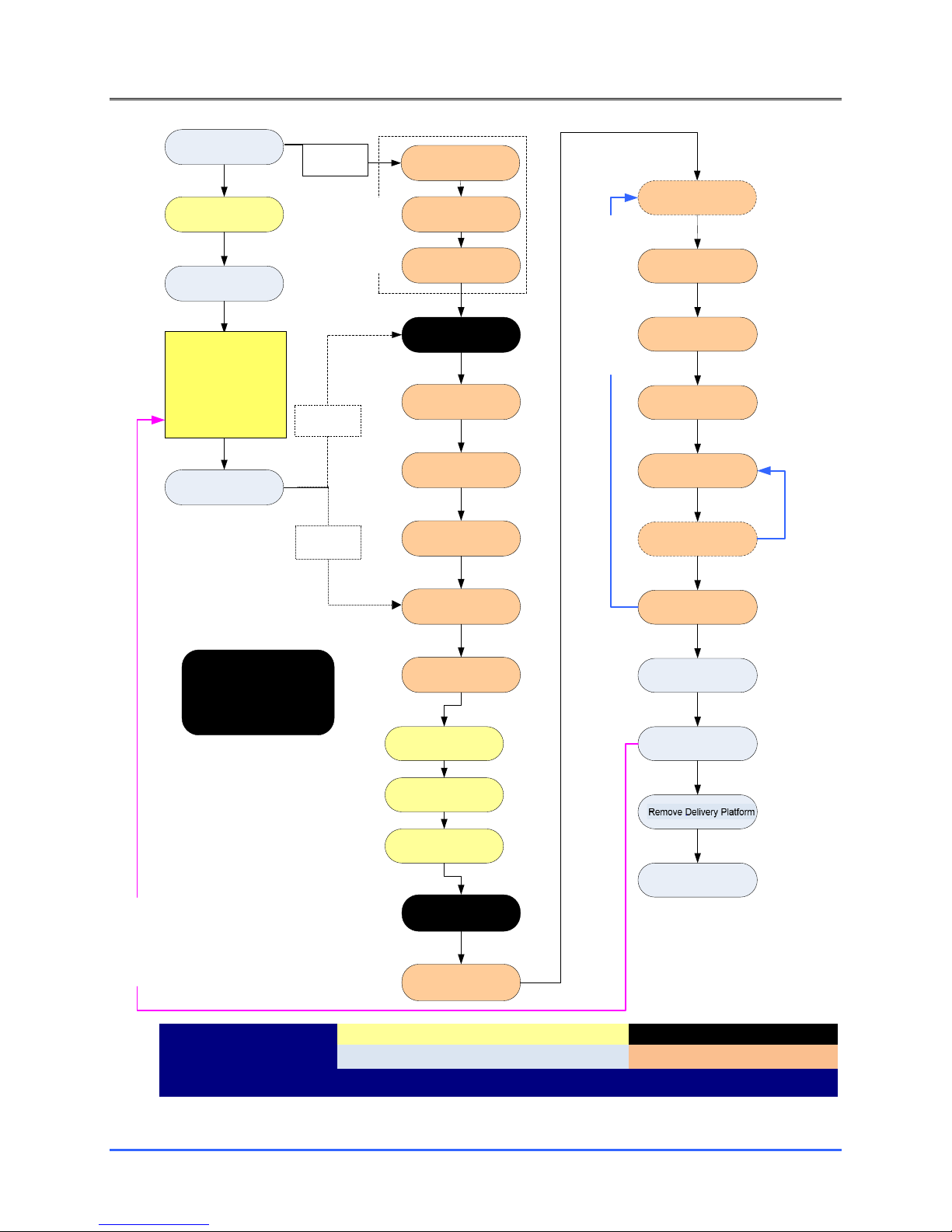
8. NEUROBLATE® SYSTEM PROCEDURE WORKFLOW...................................................................................... 57
8.1. PROCEDURE PRE-PLANNING ............................................................................................................................57
8.1.1. M*Vision Software Start Screen ..........................................................................................................57
8.1.2. Load and Register Image Data within Plan Register Task ...................................................................58
8.1.3. Create Region of Interest (ROI) Volumes within the Plan Volumes Task .............................................61
8.1.4. Create Intended Trajectories within the Trajectory Task .....................................................................63
8.1.5. Saving a Plan........................................................................................................................................65
8.1.6. Opening a Saved Plan ..........................................................................................................................66
8.2. OPERATING ROOM:PRE-PROCEDURE PREPARATION ............................................................................................67
8.2.1. Sterile Probe Delivery Platform ............................................................................................................67
8.2.2. Anesthesia............................................................................................................................................67
8.2.3. Head Fixation.......................................................................................................................................67
8.2.4. Image-Guided Surgery (IGS) System Registration (Non-Sterile) ..........................................................67
8.2.5. Prepping and Sterile Draping for Trajectory Guidance Devices ...........................................................68
8.2.6. Image-Guided Surgery (IGS) Registration - Sterile ...............................................................................68
8.2.7. Laser Delivery Probe (LDP) Platform Attachment ................................................................................68
8.2.7.1. Skull-Mounted Trajectory Devices such as AXiiiS Stereotactic Miniframe ...........................................68
8.2.7.2. Skull Bone Anchor ................................................................................................................................68
8.2.8. Create a Pathway to the Intended Target ...........................................................................................69
8.2.9. Laser Delivery Probe (LDP) Depth Stop and Size Determination..........................................................70
8.2.9.1. AXiiiS Stereotactic Miniframe - Depth Stop and Size ...........................................................................70
8.2.9.2. Skull Bone Anchor such as Monteris Mini-Bolt - Depth Stop and Size..................................................71
8.3. PREP FOR TRANSFER TO MRI...........................................................................................................................74
8.4. INTERFACE PLATFORM (IP) ATTACHMENT/DETACHMENT–TABLE OR ATAMASTABILIZATION SYSTEM............................74
8.5. TRAJECTORY CONFIRMATION AND BEAM FIDUCIAL MARKER DETECTION ..................................................................85
8.5.1. Scan and Register Data in M*Vision using Plan Register Task ............................................................86
8.5.2. Create Region of Interest (ROI) Volumes within Plan Volumes Task....................................................86
8.5.3. Create Trajectories within Trajectories Task for AXiiiS ........................................................................87
8.5.4. Create Trajectories within Plan Trajectories Task – without AXiiiS......................................................87
8.5.5. Identify Ball Marker Selection within the Align Task ...........................................................................88