WatchPAT™ONE System 1 Operation Manual
1 GENERAL INFORMATION
This manual is part of the WatchPAT™ONE (hereafter called WatchPAT) system family of
products.
1.1 Intended Use / Indications for Use
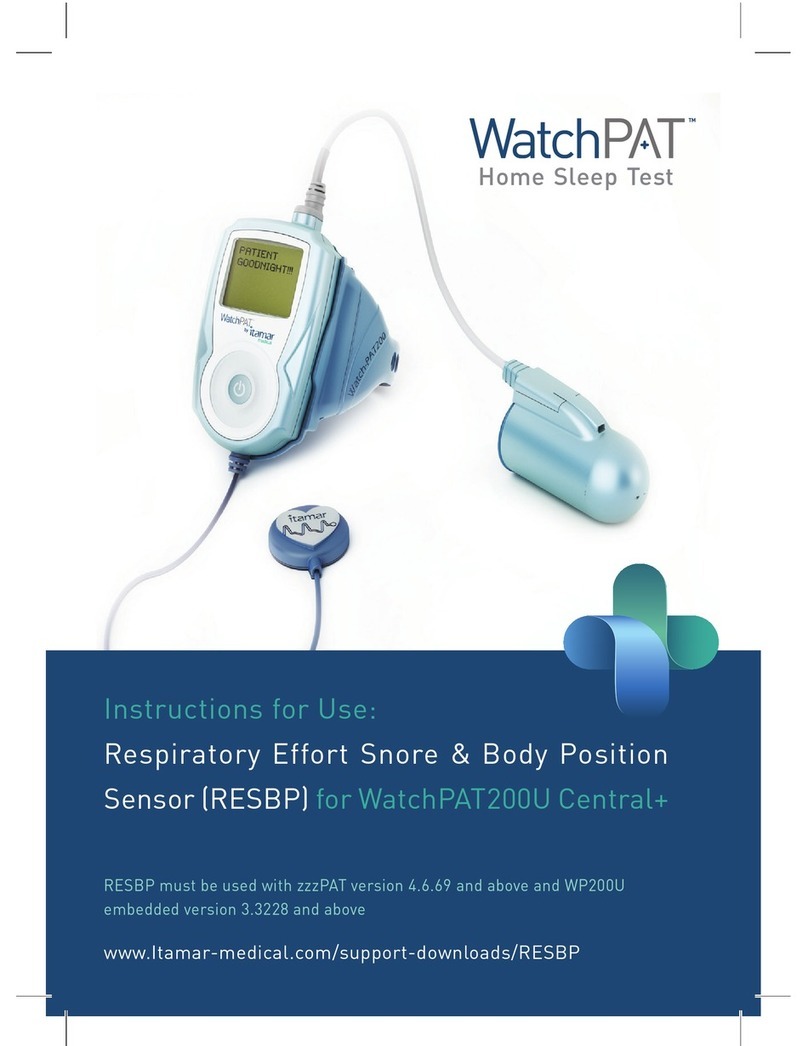
The WatchPAT™ONE (WP1) device is a non-invasive home care device for use with
patients suspected to have sleep related breathing disorders. The WP1 is a diagnostic
aid for the detection of sleep related breathing disorders, sleep staging (Rapid Eye
Movement (REM) Sleep, Light Sleep, Deep Sleep and Wake). The WP1 generates a
peripheral arterial tonometry ("PAT") Respiratory Disturbance Index ("PRDI"),
Apnea-Hypopnea index ("PAHI") and PAT sleep staging identification (PSTAGES).
WatchPAT™ONE is available in two configurations, with and without a chest sensor.
The chest sensor provides snoring level, body position and Central Apnea Hypopnea
Index (“PAHIc”).
The WP1's PSTAGES, snoring level and body position provide supplemental
information to its PRDI/PAHI/PAHIc. The WP1's PSTAGES, snoring level and body
position are not intended to be used as the sole or primary basis for diagnosing any
sleep related breathing disorder, prescribing treatment, or determining whether
additional diagnostic assessment is warranted.
PAHIc is indicated for use in patients 17 years and older. All other parameters are
indicated for 12 years and older.
1.2 Restrictions for Use
1. The WatchPAT should be used only in accordance with physician’s instructions. For
precautions see Section 1.3.
2. Only qualified medical personnel may authorize the use of the WatchPAT.
3. Qualified medical personnel must instruct the patients (and accompanying individual if
needed) how to attach and use the WatchPAT prior to use.
4. In the event of equipment malfunction all repairs should be executed by authorized
Itamar Medical Ltd. personnel or licensed service agents.
5. The eligibility of a patient for a PAT® study is entirely at the discretion of a physician
and is generally based upon the patient’s medical status.
6. The WatchPAT system in whole, or in part, may not be modified in any way.
7. The WatchPAT is used as an aid for diagnostic purposes only and should not be used
for monitoring.
8. Only suitably trained and qualified personnel should be authorized to prepare the
WatchPAT equipment prior to use.
9. The WatchPAT Operation Manual should be carefully studied by the authorized
operators and kept where it is easily accessible. Periodic review of the Manual is
recommended.
10. Itamar Medical Ltd. makes no representation whatsoever, that the act of reading the
Manual renders the reader qualified to operate, test or calibrate the system