ACUTRONIC fabian HFO User manual

Print the attached pages according to the printing instructions.
Do not print this page.
Versions
Ver. Chg. Order Description
A TBD Initial release
Vyaire Medical, Inc.
Cover Sheet
Title: Fabian HFO and Auxiliary Systems
Part no.: 113003.EN V5.1.x 2019####, Ver.: A

113003.EN V5.1.x 2019####, Version A
Ventilation Beyond Limits
fabian HFO and Auxiliary Systems
Instructions for Use, Software Version 5.1.x
ii
113003.EN V5.1.x 2019####, Version A
Disclaimer
ACUTRONIC Medical Systems, assumes no responsibility for the use or reliability of its software on equipment that is not
furnished by ACUTRONIC Medical Systems
ACUTRONIC Medical Systems makes no warranty of any kind regarding software applications that are created by the
user.
This document contains confidential and proprietary information that is protected by copyright. All rights are reserved. Any
unauthorized copying, storage, reproduction or translation of this document in any form is strictly prohibited.
This document cannot be copied, reproduced, translated, stored in a retrieval system, transmitted in any form, or reduced
to any electronic medium or machine-readable form, in whole or in part, without the written permission of ACUTRONIC
Medical Systems.
Manufacturer
ACUTRONIC Medical Systems AG
Fabrik im Schiffli
8816 Hirzel / Switzerland
Tel:
+41 44 729 70 80
Fax:
+41 44 729 70 81
e
-mail: info@acutronic-medical.ch
www.acutronic-medical.ch
For any additional parts and accessories, contact your local distributor for available items and price list.

iii
113003.EN V5.1.x 2019####, Version A
Contents:
1Introduction ............................................................................................................1
Working with the instructions .......................................................................................1
Notices and warnings...................................................................................................1
Applicable product versions .........................................................................................1
Symbols.......................................................................................................................2
2Warnings cautions and notices ............................................................................4
Always observe (fabian)...............................................................................................4
Liability for functionality ∕ damages...............................................................................7
Intended use................................................................................................................8
3System overview....................................................................................................9
Scope of delivery .........................................................................................................9
Contraindications .........................................................................................................9
fabian Front connections............................................................................................10
3.3.1 Devices with serial number prefix AI ∕AL .............................................................................10
3.3.2 Devices with serial number prefix 20 ∕AK ∕AH.....................................................................10
Rear panel .................................................................................................................11
3.4.1 Hardware with HDMI.............................................................................................................11
3.4.2 Hardware with Video in.........................................................................................................12
3.4.3 Initial hardware model...........................................................................................................13
3.4.4General hardware characteristics.........................................................................................14
4System functions and displays...........................................................................15
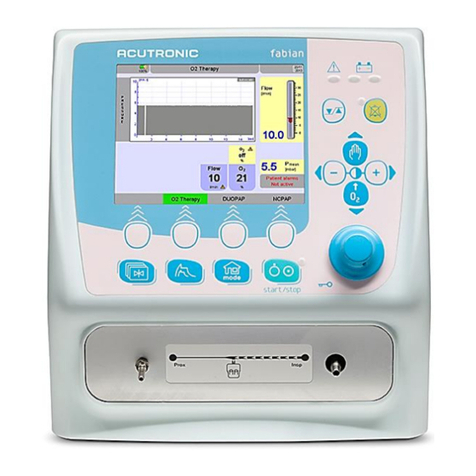
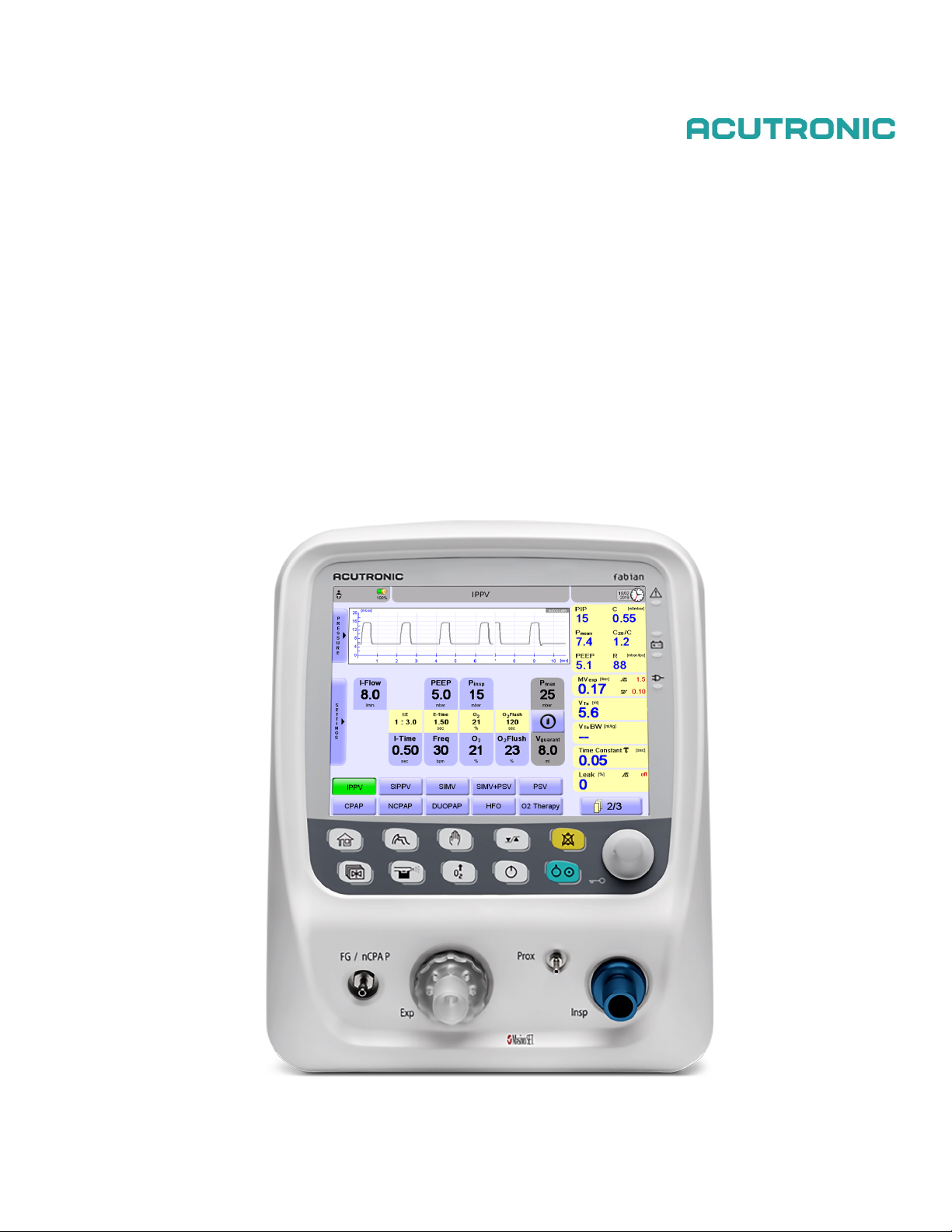
Control panel options.................................................................................................15
4.1.1 Function buttons ...................................................................................................................15
4.1.2 Rotary pulse encoder............................................................................................................16
Display concept structure...........................................................................................17
4.2.1 Display areas ........................................................................................................................17
4.2.2 Display screen ......................................................................................................................17
4.2.3 Information bar......................................................................................................................18
4.2.4 Numeric field ∕alarm limits ....................................................................................................19
4.2.5 Graphics display ...................................................................................................................21
4.2.6 LED indicators ......................................................................................................................21
Ventilation menu ........................................................................................................22
4.3.1 Operation – general ..............................................................................................................22
4.3.2 Operation – settings..............................................................................................................23
4.3.3 Ventilation parameter dependency.......................................................................................24
4.3.4 Locking ventilator parameters ..............................................................................................24
Graphics menu ..........................................................................................................26
4.4.1 Curves...................................................................................................................................27
4.4.2 Loops ....................................................................................................................................29

iv
113003.EN V5.1.x 2019####, Version A
4.4.3 Trend menu .......................................................................................................................... 30
5System operation .................................................................................................34
Preparing for operation.............................................................................................. 34
5.1.1 Connect the power supply.................................................................................................... 34
5.1.2 Connect the gas supply........................................................................................................ 34
5.1.3Connect the tubing set ......................................................................................................... 35
5.1.4 Connect Nitric Oxide (NO) tubing sets ................................................................................. 36
5.1.5 (NO) Bias flow selection ....................................................................................................... 41
Patient circuit assembly............................................................................................. 42
5.2.1 Recommended positioning of temperature probe for humidifier.......................................... 43
5.2.2 Use of reusable patient circuit.............................................................................................. 44
5.2.3 Connect nCPAP tubing set .................................................................................................. 46
5.2.4 Connect flow sensor in NIV trigger (optional) ...................................................................... 48
System start-up ......................................................................................................... 48
Device check ............................................................................................................. 50
System standby......................................................................................................... 51
System shutdown ...................................................................................................... 53
Emergency shutdown ................................................................................................ 54
6Configurations menu ...........................................................................................55
Calibration ................................................................................................................. 56
6.1.1 Flow sensor calibration ........................................................................................................ 57
6.1.2 O2Sensor calibration........................................................................................................... 60
6.1.3 etCO2module ...................................................................................................................... 61
6.1.4 SpO2module ....................................................................................................................... 61
Body weight............................................................................................................... 61
Display ...................................................................................................................... 62
6.3.1 Touch screen settings .......................................................................................................... 63
6.3.2 Trend ∕ graph display............................................................................................................ 64
Ventilation parameter settings ................................................................................... 65
Patient data ............................................................................................................... 67
Language .................................................................................................................. 67
Date ∕ Time ................................................................................................................ 68
Tools ......................................................................................................................... 68
Information ................................................................................................................ 69
Service mode ............................................................................................................ 69
7Alarms ...................................................................................................................70
Alarm limits menu ...................................................................................................... 70
7.1.1 Automatic alarm limits .......................................................................................................... 71
7.1.2 Configurable alarms ............................................................................................................. 73
Alarm log ................................................................................................................... 74
Alarm causes and solutions....................................................................................... 74
7.3.1 Alarms table ......................................................................................................................... 75

v
113003.EN V5.1.x 2019####, Version A
7.3.2 Pressure release behavior....................................................................................................82
7.3.3 Application error....................................................................................................................82
7.3.4 Watchdog alarms..................................................................................................................83
8Battery operation .................................................................................................85
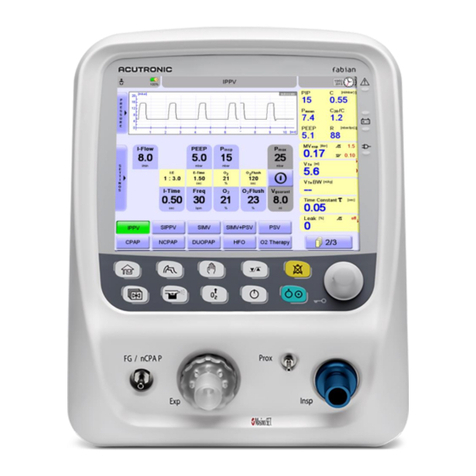
9Ventilation parameters ........................................................................................87
10 Ventilation modes ................................................................................................92
IPPV ..........................................................................................................................93
SIPPV........................................................................................................................94
SIMV..........................................................................................................................95
SIMV + PSV...............................................................................................................96
PSV ...........................................................................................................................97
CPAP.........................................................................................................................97
NCPAP ∕ DUOPAP.....................................................................................................98
O2 Therapy mode (high and low flow oxygen therapy) HFNC....................................99
HFO...........................................................................................................................99
10.9.1 HFO lung recruitment ...........................................................................................................99
10.9.2 Special HFO mode information ..........................................................................................100
Ventilation Additives ................................................................................................101
10.10.1 Volume limit ........................................................................................................................101
10.10.2 Volume guarantee ..............................................................................................................101
Special functions......................................................................................................102
10.11.1 Manual inspiration (manual Breath)....................................................................................102
10.11.2 Nebulizing medications (optional).......................................................................................102
10.11.3 O2Flush ∕ preoxygenation..................................................................................................102
11 Accessories and options................................................................................... 103
Accessories List.......................................................................................................103
CO2monitoring........................................................................................................105
11.2.1 CO2Sensor module types and selection ...........................................................................105
11.2.2 Connect the CO2module to fabian HFO............................................................................107
11.2.3 MicroPod®sensor module .................................................................................................109
11.2.4 Respironics®CO2sensors .................................................................................................116
SpO2module...........................................................................................................120
11.3.1 Setting up the Masimo sensor ............................................................................................120
11.3.2 Sensitivity mode..................................................................................................................121
11.3.3 Fast SAT mode ...................................................................................................................121
11.3.4 Alarm delay .........................................................................................................................122
11.3.5 SpO2averaging time ..........................................................................................................122
PRICO .....................................................................................................................123
11.4.1 General information on PRICO...........................................................................................123
11.4.2 Setting up PRICO ...............................................................................................................124
11.4.3 PRICO Ventilation modes...................................................................................................127

vi
113003.EN V5.1.x 2019####, Version A
11.4.4 PRICO disabling alarms ..................................................................................................... 128
11.4.5 PRICO re-enabling cases .................................................................................................. 128
11.4.6 PRICO errors...................................................................................................................... 128
FOT......................................................................................................................... 130
11.5.1 Forced Oscillation Technique (FOT) at fabian HFO .......................................................... 130
11.5.2 FOT general layout............................................................................................................. 131
11.5.3 Erasing the FOT graph....................................................................................................... 132
11.5.4 FOT disabling conditions.................................................................................................... 132
11.5.5 FOT procedure ................................................................................................................... 133
11.5.6 FOT specifications.............................................................................................................. 137
12 Ventilator service and maintenance intervals..................................................138
13 Sterilization ∕ cleaning ∕ disinfection .................................................................139
14 Setting ranges and parameters.........................................................................141
15 Guide to volume guarantee ...............................................................................145
fabian Volume guarantee (VG) operation ................................................................ 145
Initial settings........................................................................................................... 146
How to start the VG function.................................................................................... 146
Setting up the ventilator PSV+VG............................................................................ 150
16 Special procedures ............................................................................................151
17 Technical specifications ....................................................................................152
Ambient conditions .................................................................................................. 152
Monitoring................................................................................................................ 152
Measuring................................................................................................................ 153
Resistance values ................................................................................................... 154
Ventilation menu settings......................................................................................... 154
Dimensions ∕ weight................................................................................................. 155
Ratings .................................................................................................................... 155
Data storage............................................................................................................ 156
Applied parts ........................................................................................................... 156
Internal device checks ............................................................................................. 156
Gas blender function ............................................................................................... 156
Acoustic Energy....................................................................................................... 158
18 Electromagnetic compatibility statement.........................................................159
Appendix A: Glossary .......................................................................................................... 164
Appendix B: Index ................................................................................................................ 167

1 Introduction 1
113003.EN V5.1.x 2019####, Version A
1 Introduction
Working with the instructions
These instructions for use describe equipment components and their operation. These instructions are
structured so that you can step your way through the procedures and become familiar with the operation of
the ventilator.
WARNING
Carefully read the instructions for use before using the ventilator.
After you are familiar with the basic construction and operation of the ventilator you can use this manual as a
reference.
Notices and warnings
This document features three categories of notices and warnings.
WARNING:
Warnings identify conditions or practices that could result in serious adverse reactions or potential
safety hazards.
CAUTION:
Cautions identify conditions or practices that could result in damage to the ventilator or other
equipment.
NOTE:
Notes provide additional information to clarify an explanation or instruction.
Applicable product versions
This Instructions for Use is applicable for fabian HFO devices running software version 5.1: x, where (x) can
be any number.
!

2 1 Introduction
113003.EN V5.1.x 2019####, Version A
Symbols
The symbols defined in this section may appear in this document and on the equipment label or labels.
Symbol Description
Article No.
Batch code
CAUTION, refer to operator’s manual for important safety information and
precautions.
Chemical burn warning.
Dangerous voltage warning.
Data input / output RS-232.
Data input / output RS-232.
Disposal information.
DO NOT cover.
DO NOT stack no more than 2 on top
DO NOT use hooks.
Explosion Hazard warning
External power supply Input
Flammability Fire hazard warning.
Sensor
Flow sensor connection.
Flow sensor connection.
Fragile, Handle with care.
High Frequency interference warning.
Keep away from heat.
!
!
2

1 Introduction 3
113003.EN V5.1.x 2019####, Version A
Symbol Description
Keep dry.
Manufactured without the use of natural latex or derivatives.
Manufacturer
Marking per Medical Devices Directive 93/42/EEC.
Nebulizer (Obsolete)
Network Ethernet connection.(Disabled)
Non-Sterile
NOTE symbol
Nurse Call signal output.
Nurse Call signal output.
Potential equalization connection.
Protective Earth ground.
Single use.
This way UP.
Type BF application applied part.
Type B application part
Unplug power before opening housing.
Video Output
USB connection.
Warning regarding operation in explosive areas.
4 2 Warnings cautions and notices
113003.EN V5.1.x 2019####, Version A
2 Warnings cautions and notices
Always observe (fabian)
# Symbol Description
1
NOTE:The use of the ventilator requires detailed knowledge and the
understanding of this operator’s manual. This device is only intended for the
described use.
2
WARNING:Only use this ventilator in combination with an external
monitoring device (for example: SpO2).
3
WARNING:Only operate the ventilator with accessories recommended by
ACUTRONIC Medical Systems AG.
4
WARNING:The ventilator must be operated by qualified technical staff to
ensure immediate remedial action in the event of malfunction.
5
WARNING: The fabian system and associated auxiliary systems must
NEVER be used in MRI scanning events.
6
WARNING:An alternate ventilation method (for example. resuscitation) must
always be available when using the ventilator.
7
WARNING:DO NOT use the ventilator in combination with flammable gases
or narcotic agents to prevent the risk of fire or explosion.
8
WARNING:NEVER use the ventilator in explosive environments.
9
WARNING:An audible signal indicates a system or patient alarm and always
requires action by a trained medical professional.
10
WARNING:If an alarm condition (other than the exceptions listed within this
manual) occurs while the audible alarm Silence function is engaged, only the
visual alarm indications are displayed.
11
WARNING:DO NOT silence an audible alarm, engage the audible Alarm
Silence function, or decrease the audible alarm volume if patient safety could
be compromised.
12
WARNING:DO NOT obstruct the speaker.
Blocking the speaker can result in an inaudible alarm tone.
13
WARNING:Carefully route patient cabling to reduce the risk of patient
entanglement or strangulation.
14
WARNING:NEVER connect the ventilator to patients if an error or
malfunction is detected during equipment check.
15
WARNING:NEVER connect to electrical devices not mentioned in this
operator’s manual without first consulting the manufacturer.
16
WARNING:NEVER operate the ventilator while covered or set up in a way
to negatively impact the operation or function.

2 Warnings cautions and notices 5
113003.EN V5.1.x 2019####, Version A
# Symbol Description
17
WARNING:Always unplug the ventilator from the power source before
opening the housing.
18
WARNING:NEVER use anti-static or electrically conductive tubing.
19
NOTE: The safety and health of the operators are guaranteed by the fact that
the products for example: DO NOT contain any allergenic or mutagenic
materials such as phthalates.
20
WARNING:The device can only be isolated from the main power supply by
removing the power cord completely.
•Ensure the power socket is always accessible for disconnection.
•DO NOT disconnect the power cable unless for Service purposes or
transport.
21
WARNING:DO NOT modify the equipment.
22
WARNING:Before applying non-original accessories, ensure that they are
biocompatible.All accessories supplied by ACUTRONIC Medical Systems
for use on fabian ventilators are biocompatible.
23
WARNING:When connected to a patient DO NOT simultaneously touch the
external power supply cord and the flow sensor connector cable.
24
WARNING:if the strength of the auditory alarms is less than the ambient
sound this might impede an operator to recognize alarm conditions.
25
WARNING:NEVER cover the ventilator.
26
WARNING:DO NOT position the ventilator in such a way that adversely
affects its performance or makes it difficult to disconnect the ventilator from
the mains supply. In case of emergency, removal of the mains plug from the
wall outlet disconnects the ventilator from mains power on all poles
simultaneously.
27
WARNING:in case of ventilator failure, the lack of immediate access to
appropriate alternative means of ventilation can result in patient death.
28
WARNING:Ensure that alarms are appropriately set before use of ventilator
on a patient.
29
WARNING:In case portions of the gas pathways through the VENTILATOR
become contaminated with body fluids or expired gases during NORMAL
CONDITION or SINGLE FAULT CONDITION, immediately contact
ACUTRONIC Medical Systems
30
WARNING:
•When selecting the neonatal patient size, a Neonatal Flow sensor must
be used.
•When selecting the pediatric patient size, a Pediatric Flow sensor must
be used.
31
NOTE: In general, it should be noted that ventilation of children should only
be carried out by clinically trained specialists who have sufficient knowledge
of ventilation of patients of specified age.

6 2 Warnings cautions and notices
113003.EN V5.1.x 2019####, Version A
# Symbol Description
32
WARNING: DO NOT use the etCO2module in the presence of flammable
anesthetics or other flammable substances in combination with air, oxygen-
enriched environments, or nitrous oxide.
33
WARNING:Check alarm limit settings each time the etCO2module is used.
34
WARNING:The etCO2module is intended only as an adjunct in patient
assessment. It must be used in conjunction with assessment of clinical signs
and symptoms.
35
Before use, carefully read the following literature:
•Oximetry Sensor Directions for Use
•PC-Series Patient Cable Directions for Use
36
EXPLOSION HAZARD:DO NOT use the oximeter in the presence of
flammable anesthetics or other flammable substances in combination with
air, oxygen-enriched environments, or nitrous oxide.
37
Check alarm limit settings each time the oximeter is used.
38
An oximeter should NOT be used as an Apnea monitor.
39
An oximeter should be considered an early warning device. As a trend
towards patient deoxygenation is indicated, blood samples should be
analyzed by a laboratory co-oximeter to completely understand the patient's
condition.
40
Always remove the sensor from the patient and completely disconnect the
patient from the oximeter before bathing the patient.
41
DO NOT use malfunctioning equipment. Have the unit repaired by Masimo or
a qualified service person.
42
ELECTRIC SHOCK HAZARD: DO NOT remove the oximeter cover. There
are no user-serviceable items inside the oximeter. An operator may only
perform maintenance procedures specifically described in this manual.
Measure the leakage current whenever an external device is connected to
the serial or analog output ports. Leakage current must not exceed 100 µA.
43
If the accuracy of any measurement by the oximeter does NOT seem
reasonable, first check the patient's vital signs by alternate means, and then
check the oximeter for proper functioning.

2 Warnings cautions and notices 7
113003.EN V5.1.x 2019####, Version A
Maintenance
The fabian HFO device is a ventilator classified as Class IIb according to the European Directive, as such:
•Inspection according to manufacturer specifications is required every 12 months.
•Maintenance must be performed by ACUTRONIC Medical Systems trained personnel with access to
appropriate test and measuring equipment.
ACUTRONIC Medical Systems AG representatives are strongly recommend for service agreements and
repairs.
Only use original ACUTRONIC Medical Systems parts for repairs.
Note chapter “12:Ventilator service and maintenance intervals”.
Liability for functionality ∕ damages
In the event of improper equipment maintenance or repairs by any persons not associated with ACUTRONIC
Medical Systems AG, Service or improper use, all liability for the functionality is transferred to the owner or
operator.
ACUTRONIC Medical Systems AG, assumes no liability for damages caused by the non-observance of
preceding notices. The preceding notices DO NOT extend the warranty and liability terms of the
ACUTRONIC Medical Systems AG, sales terms and delivery conditions.

8 2 Warnings cautions and notices
113003.EN V5.1.x 2019####, Version A
Intended use
The fabian HFO is intended for premature infants, new-borns as well as children weighing up to 30 kg.
The fabian HFO is intended for “in-patient use” in hospitals, medically-used rooms and intra-hospital
patient transport.
The fabian HFO is an electronically microprocessor-controlled ventilator.
The fabian HFO ventilates with excess pressure based on the continuous-flow principle.
(Time cycled, pressure ∕volume limited, or volume guaranteed)
Oxygen is metered by the integrated Air ∕O2blender.
The oxygen concentration is measured internally with a galvanic oxygen sensor.
The ventilator is intended for the following ventilation methods:
•Continuous Positive Airway Pressure (CPAP)
•High and Low flow oxygen therapy (HFNC: O2Therapy)
•High Frequency Oscillation (HFO)
(membrane principle)
•Intermittent Positive Pressure Ventilation (IPPV)
•Pressure Support Ventilation (PSV)
•Synchronized Intermittent Mandatory Ventilation (SIMV)
•Synchronized Intermittent Mandatory Ventilation combined with PSV (SIMV + PSV)
•Synchronized Intermittent Positive Pressure Ventilation (SIPPV)
•Ventilation nCPAP ∕DUOPAP with variable flow generators (NIV)
(Infant Flow™, Infant Flow™ LP, Inspire™, Medijet®)
The equipment is operated by a physician or by a physician’s orders by a professional with technical training
in this task, any operator must be trained on this equipment, be familiar with the operator’s manual and have
knowledgeable use of the equipment.
fabian HFO is NOT approved for use in a homecare environment.

3 System overview 9
113003.EN V5.1.x 2019####, Version A
3 System overview
Scope of delivery
The fabian HFO product includes the following items:
•One fabian HFO Ventilator
•One Accessory kit
•One Flow Sensor (reusable)
•One Flow Sensor Cable
•One Test Lung
•Two Infant Flow Ventilator Tubes
•One Power Cable (# country specific)
•One Operating Manual (# country specific)
Contraindications
Severe airflow obstruction and intracranial-hypertension would contraindicate the use of the fabian HFO
neonatal and infant ventilator.
In the event of ventilation for several hours or more, care must be taken for optimal conditioning of the
respiratory gases (warmth, humidification) to optimize secretion mobilization and prevent damage to mucous
membranes.

10 3 System overview
113003.EN V5.1.x 2019####, Version A
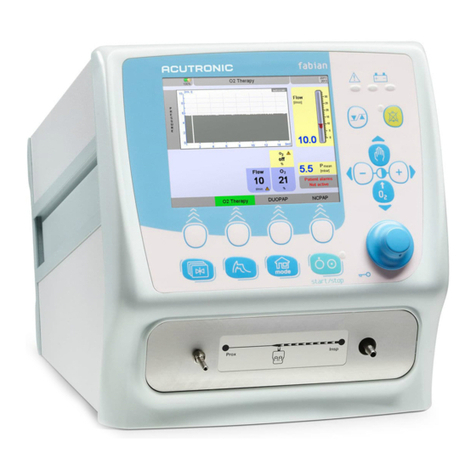
fabian Front connections
3.3.1 Devices with serial number prefix AI ∕AL
1 External Bias Flow (FG - Fresh Gas) port and port for nCPAP system based on flow
generators (single limb systems)
2 Expiratory limb port
3 Proximal Pressure port
4 Inspiratory limb port/ HFO port
3.3.2 Devices with serial number prefix 20 ∕AK ∕AH
1 Inspiratory Limb port/ center port for connecting nCPAP system based on flow
generators (single limb systems)
2 Expiratory limb port
3 Proximal Pressure port
4 HFO Port
1
2
3
4
1
2
3
4

3 System overview 11
113003.EN V5.1.x 2019####, Version A
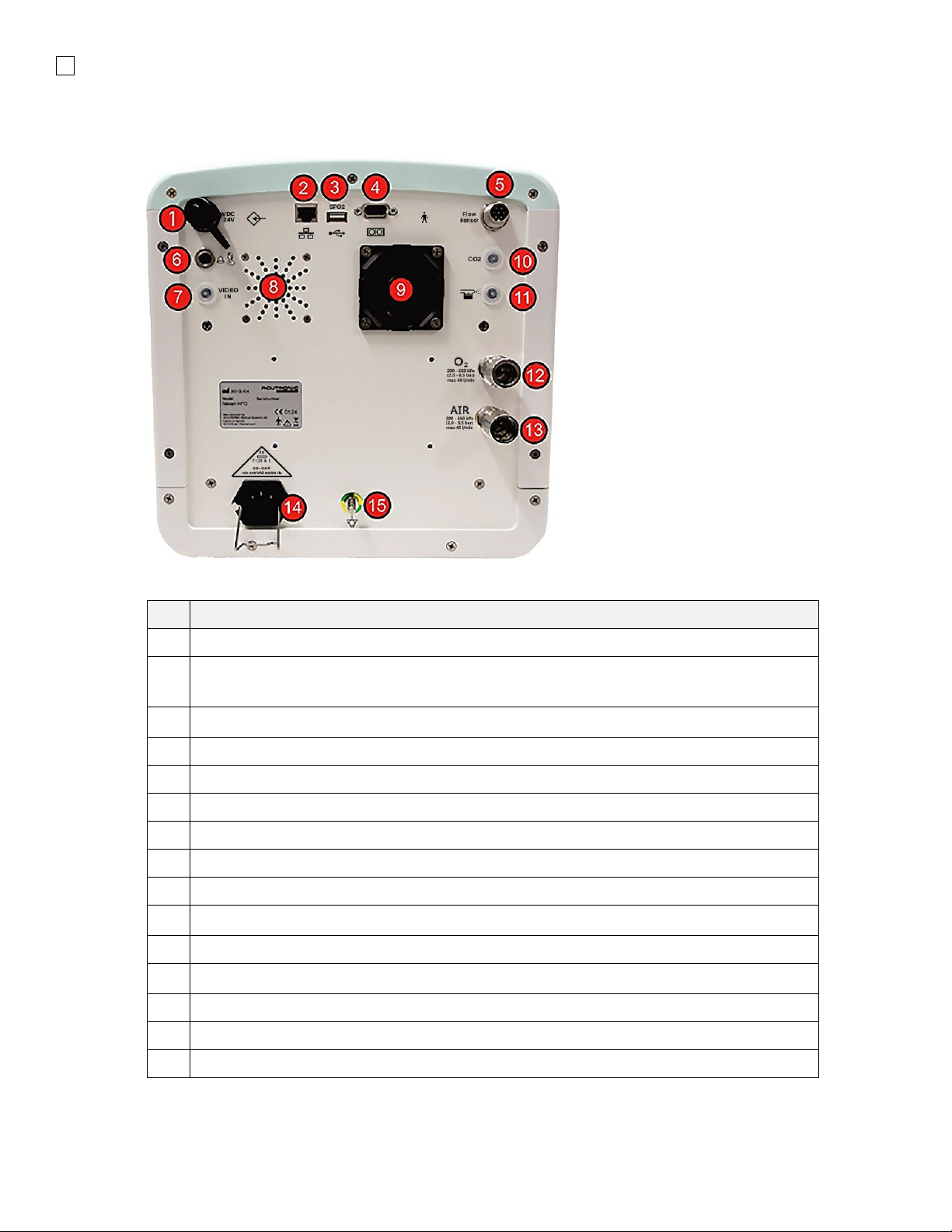
Rear panel
3.4.1 Hardware with HDMI
# Description
1 Video Out HDMI connection
2 Nebulzer & Service USB Connection
3 Network jack for data management, PDMS
(For connection to network with minimum 3 KV galvanic isolation) (DISABLED)
4 DB9 RS-232 port for PDMS
5 Flow Sensor 7-pin Connector
6 Nurse Call 3-pin Connector
7 Loudspeaker (Audio)
8 Fan
9 CO2Sensor 7-pin connection
10 SpO2Sensor 5-pin connection
11 O2Supply Connector 2.0 to 6.0 bar ∕40 Lpm
12 Pressurized Air Connector 2.0 to 6.0 bar ∕40 Lpm
13 Power Connector 100 A (fuse 1.25A L 250V)
14 Equipotential connection
1
2
3
4
5
6
7
8
9
10
11
12
13
14
12 3 System overview
113003.EN V5.1.x 2019####, Version A
3.4.2 Hardware with Video in
# Description
1 Connector for 24V DC external power supply (No charging)
2 Network jack for data management, PDMS
(For connection to network with minimum 3 KV galvanic isolation) (DISABLED)
3 USB port for data output, Software update and connection for Masimo SpO2module.
4 DB9 RS-232 port for PDMS
5 Flow Sensor 7-pin Connector
6 Nurse Call 3-pin Connector
7 Video In, VGA (NOT USED)
8 Loudspeaker (Audio)
9 Fan
10 CO2sensor (Optional)
11 Nebulizer (Not used)
12 O2supply connector 2.0 to 6.5 bar ∕40 l/min
13 Pressurised air connector 2.0 to 6.5 bar ∕40 l/min
14 Power Connector (fuse 1.25 AT)
15 Equipotential connection
Other manuals for fabian HFO
1
Table of contents
Other ACUTRONIC Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Flame
Flame NebulAIR+ Instructions for use manual

Precision Medical
Precision Medical PM Series user manual

Braun
Braun Draina S Large Instructions for use

Select Medical
Select Medical OLA 8 user manual

Saviour Medical
Saviour Medical TECHNICAL STRETCHER Information

Diaglobal
Diaglobal Duo Photometer plus DP 210 operating manual

InspireMD
InspireMD CGUARD CRX0620 Instructions for use

Mackworth
Mackworth HOLLY User instructions

OstomyCure
OstomyCure TIES Care manual

Mediroyal
Mediroyal MR4996 instructions
Invacare
Invacare Aquatec Ocean VIP user manual

Drive DeVilbiss Healthcare
Drive DeVilbiss Healthcare CHAD CH4808-L-BLUE PRODUCT INFORMATION AND INSTRUCTIONS