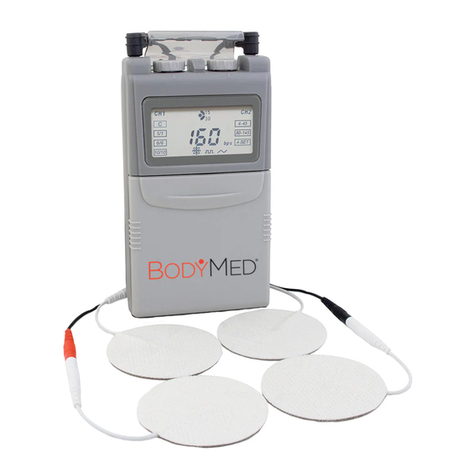
BodyMed ZZACOMBOBDPT2 User manual

TENS/EMS/Massager Combo
ZZACOMBOBDPT2
Please read this instruction manual
prior to operating.

2
All Rights Reserved.
ZZACOMBOBDPT2 - Rev. 2019
CAUTION: Federal law restricts this device to sale
by or on the order of a physician.

3
Table of Contents
1. Foreword .........................................................................4
2. Safety Information...........................................................6
3. Getting to Know Your Device.........................................13
4. Specication..................................................................15
5. Operating Instruction....................................................19
6. Instructions for Use ......................................................23
7. Cleaning and Maintenance ...........................................29
8. Troubleshooting ............................................................32
9. Storage..........................................................................33
10. Disposal.......................................................................33
11. Electromagnetic Compatibility (EMC) Tables .............34
12. Normalized Symbols...................................................38
13. Warranty......................................................................39

4
1. FOREWORD
Introduction
The device ZZACOMBO is a dual channel output TENS,
EMS, and MASSAGE stimulator. Before using, please read
the instruction manual.
The COMBO stimulator belongs to the group of electrical
stimulation systems. It has three basic functions: TENS
(Transcutaneous Electrical Nerve Stimulation), EMS
(Electronic Muscle Stimulation), and MASSAGE.
Function of the COMBO stimulator: The device has 60
programs (30 TENS programs, 27 EMS programs, and 3
MASSAGE programs) and applies electric currents in the
low-frequency range for therapy. Each program controls
the generated electric impulses, their intensity, frequency,
and pulse width.
Based on simulating the body’s natural pulses, the
mechanism of electrical stimulation equipment is to create
electric impulses that are transcutaneously transmitted
to nerves or muscle bers through the electrode.
The intensity of the dual channel can be adjusted
independently and applied individually to one body part.
This dual channel device can be used with four pieces of
electrodes, which allow you to stimulate different muscle
groups simultaneously with a wide selection of standard
programs. The electrical pulse is rst transmitted to the
tissue, then it affects the transition of stimulation in nerves
as well as muscle tissues in the body parts.

5
1.2 Medical Background
1.2.1 About Pain
Pain is an important signal in the human body warning
system. It reminds us that something is wrong without
which abnormal conditions may go undetected, causing
damage or injury to vital parts of our bodies. Even
though pain is a necessary warning signal of trauma or
malfunction in the body, nature may have gone too far in its
design. Aside from its function in diagnosis, long-lasting
persistent pain is not useful to its original purpose.
Pain does not occur until an encoded message travels to
the brain where it is decoded, analyzed, and reacted to
from the injured area along the small nerves leading to the
spinal cord. There the message is transmitted to different
nerves that travel up the spinal cord to the brain. Then the
pain message is interpreted, referred to, and pain is felt.
1.2.2 What Is TENS?
TENS (Transcutaneous Electrical Nerve Stimulation) is
effective in relief of pain. It is used daily and clinically
proven by physiotherapists, caregivers, and top athletes
around the world. High-frequency TENS currents activate
the pain-inhibiting mechanisms of the nervous system.
Electrical impulses from electrodes, placed on the
skin over or near the pain area, stimulate the nerves to
block the pain signals to the brain, causing the pain go
unperceived. Low-frequency TENS currents facilitate the
release of endorphins, the body’s natural painkillers.

6
1.2.3 What Is EMS?
Electrical Muscle Stimulation is an internationally
accepted and proven way of treating muscular injuries. It
works by sending electronic pulses to the muscle needing
treatment that causes the muscle to exercise passively. It
is a product deriving from the square waveform, originally
invented by John Faraday in 1831. Through the square
wave pattern, it is able to work directly on muscle motor
neurons. The EMS System has low frequencies and this
in conjunction with the square wave pattern allows direct
work on muscle groupings.
2. SAFETY INFORMATION
2.1 Intended Use
TENS Mode
• Symptomatic relief of chronic intractable pain
• Post traumatic pain
• Post surgical pain
EMS Mode
• Relaxation of muscle spasms
• Prevention of disuse atrophy
• Increasing local blood circulation
• Muscle re-education
• Immediate post-surgical stimulation of calf muscles to
prevent venous thrombosis
• Maintaining or increasing range of motion

7
2.2 Important Safety Precautions and Warnings
It is important that you read all the warnings and
precautions included in this manual because they are
intended to keep you safe, prevent risk of injury, and
avoid a situation that could result in damage to the device.
SAFETY SYMBOLS USED IN THIS MANUAL
2.2.1 Contraindication
1) Do not use this device if you are using a
cardiac pacemaker, implanted debrillator,
or other implanted metallic or electronic
devices. Such use could cause electric shock,
burns, electrical interference, or death.
2) The device should not be used when cancerous lesions or
other lesions are present in the treatment area.
3) Stimulation should not be applied over swollen,
infected, inamed areas or skin eruptions (e.g. phlebitis,
thrombophlebitis, varicose veins, etc.).
4) Electrode placements must be
avoided in the carotid sinus area
(anterior neck) or transcerebrally
(through the head).
5) This device should not be used in
overly enervated areas.
6) This device should not be used on an inguinal hernia.
7) Do not use on scarred areas following a surgery for at least
10 months after the operation.
8) Do not use with serious arterial circulatory problems in the
lower limbs.

8
2.2.2 Warning
1) The long-term effect electrical stimulation is unknown.
Electrical stimulation devices do not have any curative value.
2) This device should only be used under the continued
supervision of a licensed medical practitioner.
3) Electrical stimulation is not effective for central origin pain
such as headache.
4) Stimulation should not be applied over the carotid sinus
nerves, particularly in patients with a known sensitivity to the
carotid sinus reex.
5) Stimulation should not be applied over the neck or mouth.
Severe spasm of the laryngeal and pharyngeal muscles may
occur and the contractions may be strong enough to close
the airway or cause difculty in breathing, or adverse effects
on heart rhythm or blood pressure.
6) Do not apply stimulation in the presence of electronic
monitoring equipment (e.g., cardiac monitors, ECG alarms),
which may not operate properly when electrical stimulation
device is in use.
7) Do not apply stimulation across the patient’s chest because
the introduction of electrical current into the chest may cause
rhythm disturbances to the patient’s heart, which could be
lethal.
8) Do not apply stimulation over open wounds or rashes, or over
swollen, red, infected, or inamed areas or skin eruptions (
e.g. phlebitis, thrombophlebitis, varicose veins).
9) Do not apply stimulation while the patient is sleeping.
10) Stimulation should not be applied transthoracically in that
the introduction of electrical current into the heart may cause
cardiac arrhythmias.

9
11) Stimulation should not take place while the user is
connected to high-frequency surgical equipment, it may
cause burn injuries on the skin under the electrodes, as well
as problems with the stimulator.
12) Do not use the stimulator in the vicinity of shortwave or
microwave therapy equipment, since this may
affect the output power of the stimulator.
13) Never use in environments with high humidity
such as in the bathroom or when having a bath
or shower.
14) Never use near the heat. Stimulation electrodes should
never be placed anywhere on the front of the thorax (marked
by ribs and breastbone),but above all not on
the two large pectoral muscles. Here it can
increase the risk of ventricular brillation and
lead to cardiac arrest.
15) Never use on the eye area.
16) Never use near the genitals.
17) Apply the electrodes to clean, dry, and unbroken skin only.
18) Keep electrodes separate during treatment, electrodes in
contact with other could result in improper stimulation or
skin burns.
19) Consult your doctor if you are in any doubt whatsoever.
20) Consult with your doctor before using this device, because
the device may cause lethal rhythm disturbances to the heart
in susceptible individuals.
21) Discontinue and do not increase the intensity level if you feel
discomfort during use.
22) Secure limbs (e.g. arms, legs) in place during arm and leg
stimulation.
23) Avoid standing during leg stimulation to prevent falls.

10
2.2.3 Precautions
1) For single patient use only.
2) Keep yourself informed of the contraindications.
3) This stimulator shall never be used by patients who have
noncompliant, emotionally disturbed, dementia, or low IQ.
4) Read, understand, and practice the precautionary and
operating according to the instructions. Know the
limitations and hazards associated with using any other
device. Observe the precautionary and operational decals
that placed on the unit.
5) The instruction of use was listed; any improper use may be
dangerous.
6) Caution should be used for patients who are suspected or
diagnosed with heart problems.
7) Some patients may experience skin irritation or hypersensitivity
due to the electrical stimulation or silicone rubber. If rash
develops or pain persists, discontinue use and consult a doctor.
The irritation can usually be reduced by using an alternate
conductive medium or alternate electrode placement.
8) Electrode placement and stimulation settings should be
based on the guidance of prescribing practitioner.
9) Effectiveness is highly dependent upon patient selection
by a person qualied in the management of pain-aficted
patients.
10) Isolated cases of skin irritation may occur at the site of the
electrode placement following long-term application.
11) The electrodes can only be placed on healthy skin. Avoid
skin irritation by ensuring that good contact is achieved
between electrodes and skin.
12) If the stimulation levels are uncomfortable or become
uncomfortable, reduce the stimulation intensity to a
comfortable level and contact your physician if problems
persist.

11
13) Do not apply stimulation while the patient is driving,
operating machinery, or during any activity in which
electrical stimulation can put the patient at risk of injury.
14) Never use the device in rooms where aerosols (sprays) are
used or pure oxygen is being administered.
15) Do not use it near any highly-ammable substances,
gases, or explosives.
16) Do not use this device at the same time as other
equipment, which sends electrical pulses to your body.
17) Do not confuse the electrode cables and contacts with
your headphones or other devices, and do not connect the
electrodes to other devices.
18) Do not use sharp objects such as pencil point or ballpoint
pen to operate the buttons on the control panel.
19) Inspect applicator cables and associated connectors before
each use.
20) Electrical stimulators should be used only with the leads
and electrodes recommended by the manufacturer.
21) Use this device only with the leads, electrodes, and
accessories recommended by the manufacturer.
22) TENS is not a substitute for pain medications and other
pain management therapies.
23) TENS is a symptomatic treatment and as such suppresses
the sensation of pain that would otherwise serve as a
protective mechanism.
24) Since the effects of stimulation of the brain are unknown,
stimulation should not be applied across the head, and
electrodes should not be placed on opposite sides of the
head.

12
2.2.4 Adverse Reactions
1) Possible skin irritation or electrode burn under the
electrodes may occur.
2) Possible allergic skin reaction to tape or gel may occur.
3) If the stimulation levels are uncomfortable or become
uncomfortable, reduce the stimulation intensity to a
comfortable level and contact your physician if problems
persist.
4) Patients may experience skin irritation and burns beneath
the stimulation electrodes applied to the skin.
5) Patients may experience headache and other painful
sensations during or following the application of electrical
stimulation near the eyes and to the head and face.
6) Patients should stop using the device and should consult
with their physicians if they experience adverse reactions
from the device.
25) Use caution when the patient has a tendency to bleed
internally, such as following an injury or fracture.
26) Safety of powered muscle stimulators for use during
pregnancy has not been established.
27) Caution should be used for patients with suspected or
diagnosed epilepsy.
28) Caution should be used in the presence of the following:
29) When there is a tendency to hemorrhage following acute
trauma or fracture;
30) Following recent surgical procedures when muscle
contraction may disrupt the healing process;
31) Over the menstruating or pregnant uterus; and
32) Over areas of the skin that lack normal sensation.

13
3. GETTING TO KNOW YOUR DEVICE
No. Function Description No. Function Description
1 Treatment mode 9 Program No. or Treatment time
2 Key locking symbol 10 Massage type
3 Treatment body part 11 SET symbol
4 Model of human body 12 Pulse rate and width symbol
5Intensity for Channel A 13 Intensity for Channel B
6 Low battery symbol 14 Symbol of Channel A
7 Timer symbol 15 Indicator Flag of Channel selection
8 Program symbol 16 Symbol of Channel B
3.2 LCD Display
1
6
2
7
3
8
9
10
11
12
13
14
5
4
15 16
No. Description QTY
1 The COMBO Stimulator 1pc
2 Electrode pad (50mm×50mm ) 4pcs
3 Electrode wires 2pcs
4 USB charging cord 1pc
5 Belt clip 1pc
6 User manual 1pc
3.1 Components Included

14
No. Description
1LCD display
2Charger indicator:
When the device is charging, the indicator light will be yellow.
When charging is completed, the indicator light will be green.
3[ON/OFF/M] button:
At power saving mode, press the [ON/OFF/M] button to turn on the device;
At standby mode, press the [ON/OFF/M] button to select the treatment
mode; press and hold the [ON/OFF/M] button to turn off the device;
At treating mode, press the [ON/OFF/M] button to stop the treatment.
4[P] button:
At standby mode, press the [S] button to enter in the setting mode.
5[+] button:
At standby or treating mode, press the [+] button to increase the intensity of CH1,
CH2 or CH1 and CH2.
6[B] button:
At standby mode, press the [B] button to select the treatment body part.
At treating mode, press and hold [B] button to turn on/off lock function.
7[CH] button:
At standby mode or treating mode, press the [CH] button to select the treatment
channel.
8[-] button:
At treating mode, press the [-] button to decrease the intensity of CH1, CH2 or
CH1 and CH2.
9 Output socket
10 USB socket
3.3 Device Illustration
1
3
25
7
4
6
8
10
9

15
Device name Combo Electrotherapy Device
Model/type ZZACOMBOBDPT2
Power sources 3.7 V Li-Ion battery
Output channel Dual channel
Waveform Bi-phase square-wave pulse
Output current Max. 120mA (at 500ohm load)
Output intensity 0 to 40 levels, adjustable
Treatment mode: TENS, EMS, and MASSAGE mode
Operating condition 5° C to 40° C with a relative humidity of 15%–93%,
atmospheric pressure from 700 hPa to 1060 hPa
Storage condition -10° C to 55° C with a relative humidity of 10%–95%,
atmospheric pressure from 700 hPa to 1060 hPa
Dimension 109*54.5*23mm (L x W x T)
Weight About 76g
Automatic Shutoff 1 minute
Classification BF type applied part, internal power equipment, IP22
Size of electrodes pad 50x50mm, square
Output precision ±20% error is allowed for all the output parameters
4. SPECIFICATION
4.1 Technical Information
MASSAGE Mode
Number of programs 3 programs
P.W. (Pulse width) 120–250μs
P.R. (Pulse Rate) 28–97Hz (Hz=vibration per second)
Treatment time 30 minutes
TENS Mode
Number of programs 30 programs
P.W. (Pulse Width) 50–300μs
P.R. (Pulse Rate) 2–120Hz (Hz=vibration per second)
Treatment time 5–90 minutes
EMS Mode
Number of programs 27 programs
P.W. (Pulse Width) 100–300μs
P.R. (Pulse Rate) 4–100Hz (Hz=vibration per second)
Treatment time 5–90 minutes (adjustable)

16
Mode Body
Part Program Pulse Rate (Hz) Pulse Width (us) Treatment Time (min) Remark
TENS
Neck
P1 80-100 120-100 Default:30
Adj.:(5-90) P.R.W.M
P2 4 150-200 Default:30
Adj.:(5-90) P.W.M
U1 Default:35
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Shoulder
P1 80-100 100 Default:30
Adj.:(5-90) P.R.M
P2 2-60 260-160 Default:30
Adj.:(5-90) P.R.W.M
U1 Default:100
Adj.:(2-100)
Default:150
Adj.:(100-300)
Default:30
Adj.:(5-90) Burst
Arm
P1 2 250 Default:30
Adj.:(5-90) Continuous
P2 100 150 Default:30
Adj.:(5-90) Burst
U1 Default:100
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Hand
P1 100 100 Default:30
Adj.:(5-90) Continuous
P2 2-10 200 Default:30
Adj.:(5-90) P.R.M
U1 Default:60
Adj.:(2-100)
Default:260
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Back
P1 60/50/45/
10/50/35 200 Default:30
Adj.:(5-90) P.R.M
P2 6/8/10 250 Default:30
Adj.:(5-90) P.R.M
U1 Default:55
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Abdomen
P1 80-120 120-100 Default:30
Adj.:(5-90) P.R.W.M
P2 120 55 Default:30
Adj.:(5-90) Continuous
U1 Default:80
Adj.:(2-100)
Default:100
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Hip
P1 100 150 Default:30
Adj.:(5-90) Burst
P2 40/6/50 200 Default:30
Adj.:(5-90) P.R.M
U1 Default:80
Adj.:(2-100)
Default:180
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
Leg
P1 40/6/50 250 Default:30
Adj.:(5-90) P.R.M
P2 80 150 Default:30
Adj.:(5-90) Continuous
U1 Default:6-10
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) P.R.M
Foot
P1 80-120 100-120 Default:30
Adj.:(5-90) P.R.W.M
P2 2-10 200 Default:30
Adj.:(5-90) P.R.M
U1 Default:2-60
Adj.:(2-100)
Default:260-160
Adj.:(100-300)
Default:30
Adj.:(5-90) P.R.W.M
Joint
P1 100 150 Default:30
Adj.:(5-90) Burst
P2 120 100-120 Default:30
Adj.:(5-90) P.W.M
U1 Default:80
Adj.:(2-100)
Default:180
Adj.:(100-300)
Default:30
Adj.:(5-90) Continuous
4.2 Treatment Programs

17
Mode Body
Part Program Pulse Rate (Hz) Pulse Width (us) Treatment Time (min) Type of
Waveform
EMS
Neck
P1 30 200 Default:30
Adj.:(5-90) Synchronous
P2 40 200 Default:30
Adj.:(5-90) Synchronous
U1 Default:50
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Shoulder
P1 45 200 Default:30
Adj.:(5-90) Synchronous
P2 55 200 Default:30
Adj.:(5-90) Synchronous
U1 Default:80
Adj.:(2-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Arm
P1 50 150 Default:30
Adj.:(5-90) Synchronous
P2 60 150 Default:30
Adj.:(5-90) Synchronous
U1 Default:80
Adj.:(20-100)
Default:150
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Hand
P1 4 200 Default:30
Adj.:(5-90) Continuous
P2 5 300 Default:30
Adj.:(5-90) Continuous
U1 Default:20
Adj.:(2-100)
Default:150
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Back
P1 60 200 Default:30
Adj.:(5-90) Synchronous
P2 70 200 Default:30
Adj.:(5-90) Synchronous
U1 Default:80
Adj.:(20-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Abdomen
P1 20 200 Default:30
Adj.:(5-90) Synchronous
P2 50 200 Default:30
Adj.:(5-90) Synchronous
U1 Default:60
Adj.:(20-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Hip
P1 30 150 Default:30
Adj.:(5-90) Synchronous
P2 60 150 Default:30
Adj.:(5-90) Synchronous
U1 Default:40
Adj.:(2-100)
Default:150
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Leg
P1 20 200 Default:30
Adj.:(5-90) Synchronous
P2 80 200 Default:30
Adj.:(5-90) Synchronous
U1 Default:25
Adj.:(20-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Foot
P1 4 200 Default:30
Adj.:(5-90) Continuous
P2 5 300 Default:30
Adj.:(5-90) Continuous
U1 Default:20
Adj.:(20-100)
Default:200
Adj.:(100-300)
Default:30
Adj.:(5-90) Synchronous
Massage
Knead P01 28-44 120~250 30 P.R.W.M
Rub P01 25-79 120~250 30 P.R.W.M
Tab P01 49-97 100~240 30 P.R.W.M

18
4.3 The waveform of the stimulation program:
Continuous
P.W.M (Pulse Width Modulation)
P.R.M (Pulse Rate Modulation)
TENS
Cycle time
Cycle time

19
Burst
EMS
Synchronous
Continuous
P.R.W.M (Pulse rate and width modulation)
Cycle tim

20
5.2 Connect Electrode Pads to Electrode Wires
Insert the electrode wires connector into the electrode connector.
Make sure they are properly connected to ensure good
performance. Please refer to the picture below.
5.2 Connect Electrode Wires to Device
Before proceeding to this step, ensure that the device is
completely switched OFF.
Hold the insulated portion of the electrode wire connector and
insert the plug into the receptacle on the top of the main device.
Ensure the electrode wires are inserted correctly. The device has
two output receptacles controlled by Channel A and Channel B
at the top of the unit. You may choose to use one channel with
one pair of electrode wires or both channels with two pairs
of electrode wires. Using both channels gives the user the
advantage of stimulating two different areas at the same time.
Caution
Caution
Always use the electrode pads that comply with the requirements
of the IEC/EN60601-1, ISO10993-1/-5/-10 and IEC/ EN60601-1-2,
as well as CE and FDA 510(K) regulation.
Do not insert the plug of the electrode wires into any AC power
supply socket.
5. OPERATING INSTRUCTION
Table of contents
Other BodyMed Medical Equipment manuals
BodyMed
BodyMed BDMDOORTC User manual

BodyMed
BodyMed ZZACOMBO User manual

BodyMed
BodyMed ZZAEV820OTC User manual

BodyMed
BodyMed ZZAN602 User manual

BodyMed
BodyMed ZZHP147 User manual

BodyMed
BodyMed zza350t User manual

BodyMed
BodyMed ZZRWAL03 User manual
BodyMed
BodyMed ZZACOMBOBDPT1 User manual

BodyMed
BodyMed ZZA500 User manual

BodyMed
BodyMed ZZA250 User manual